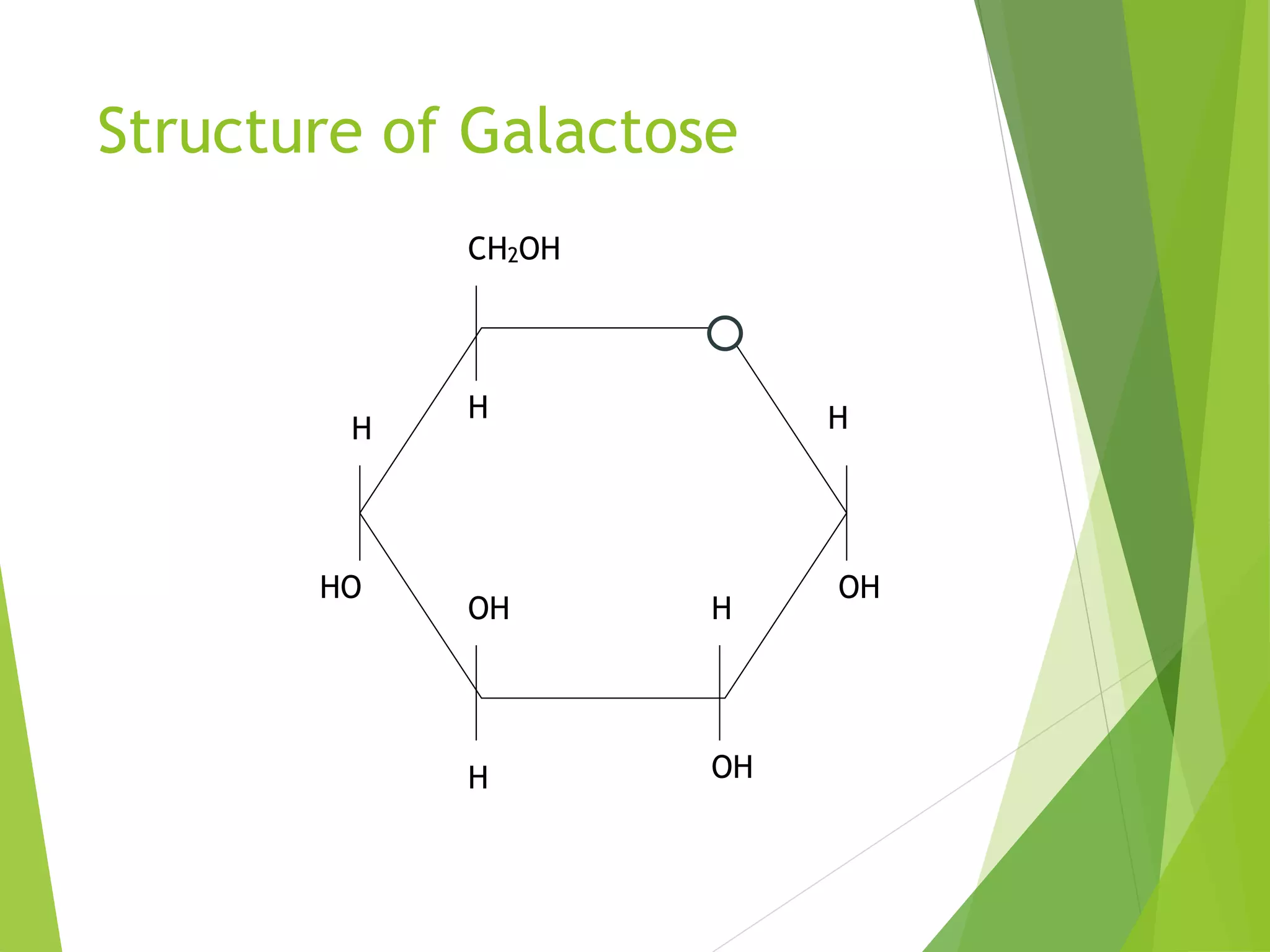
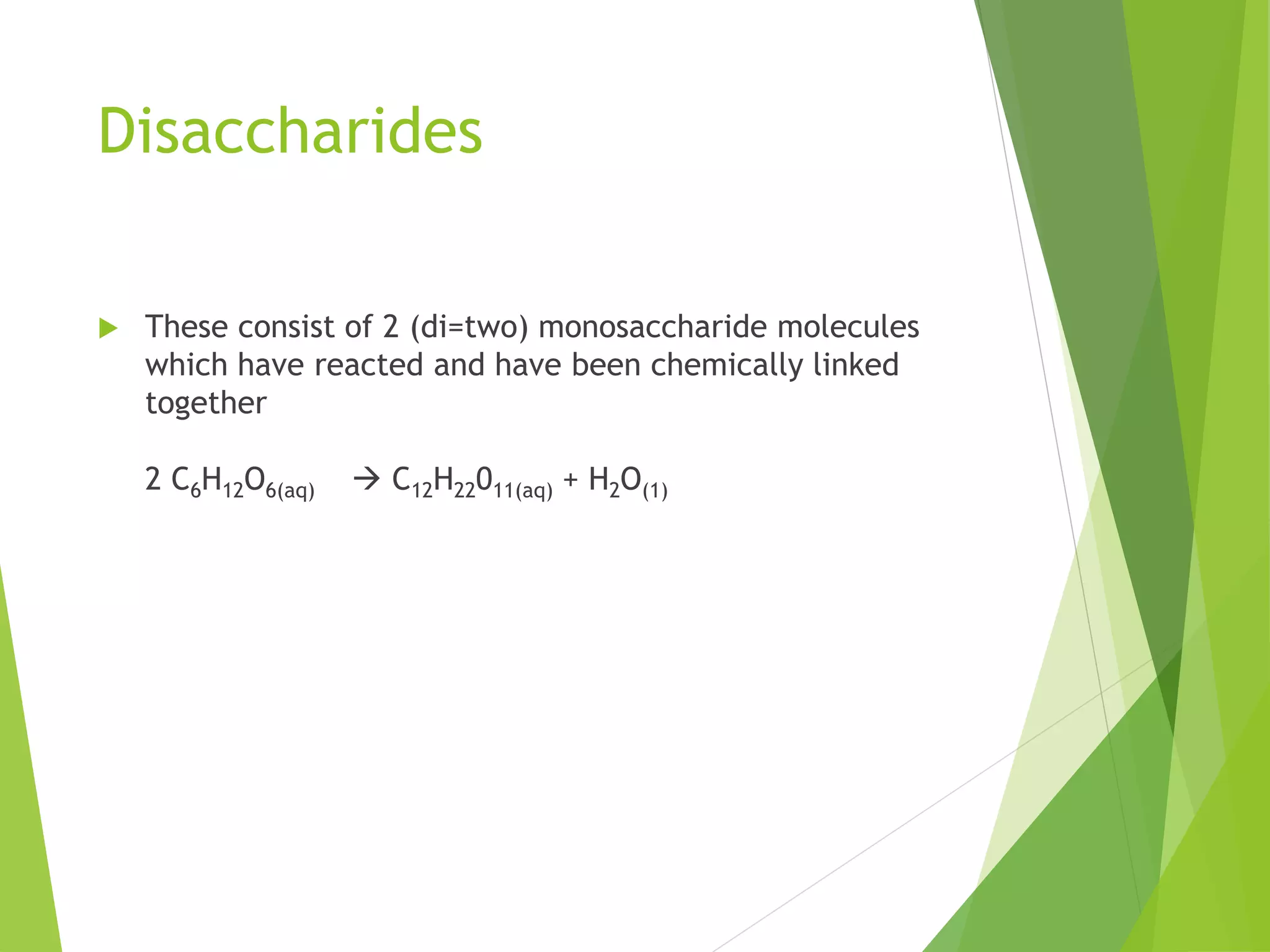
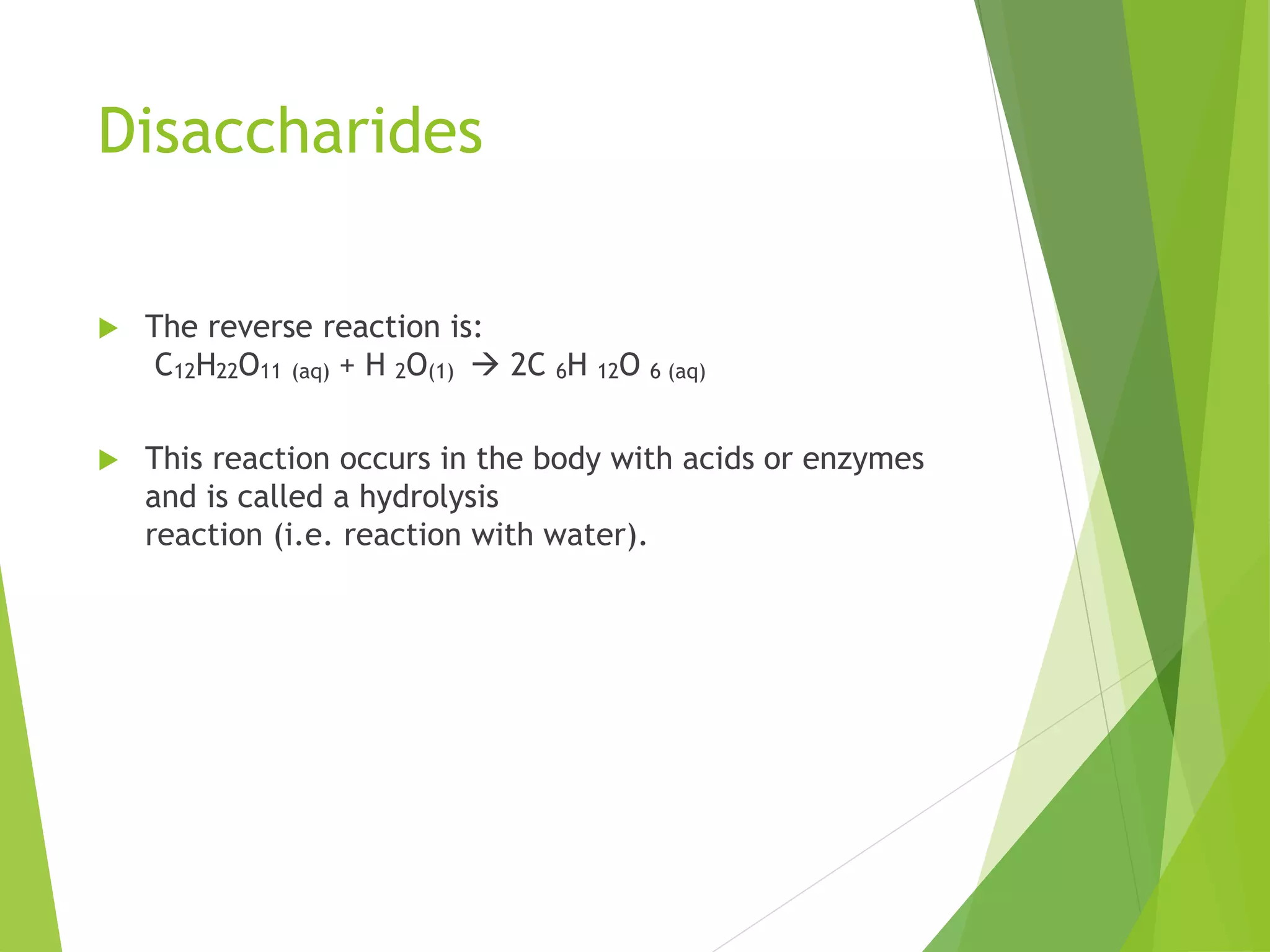
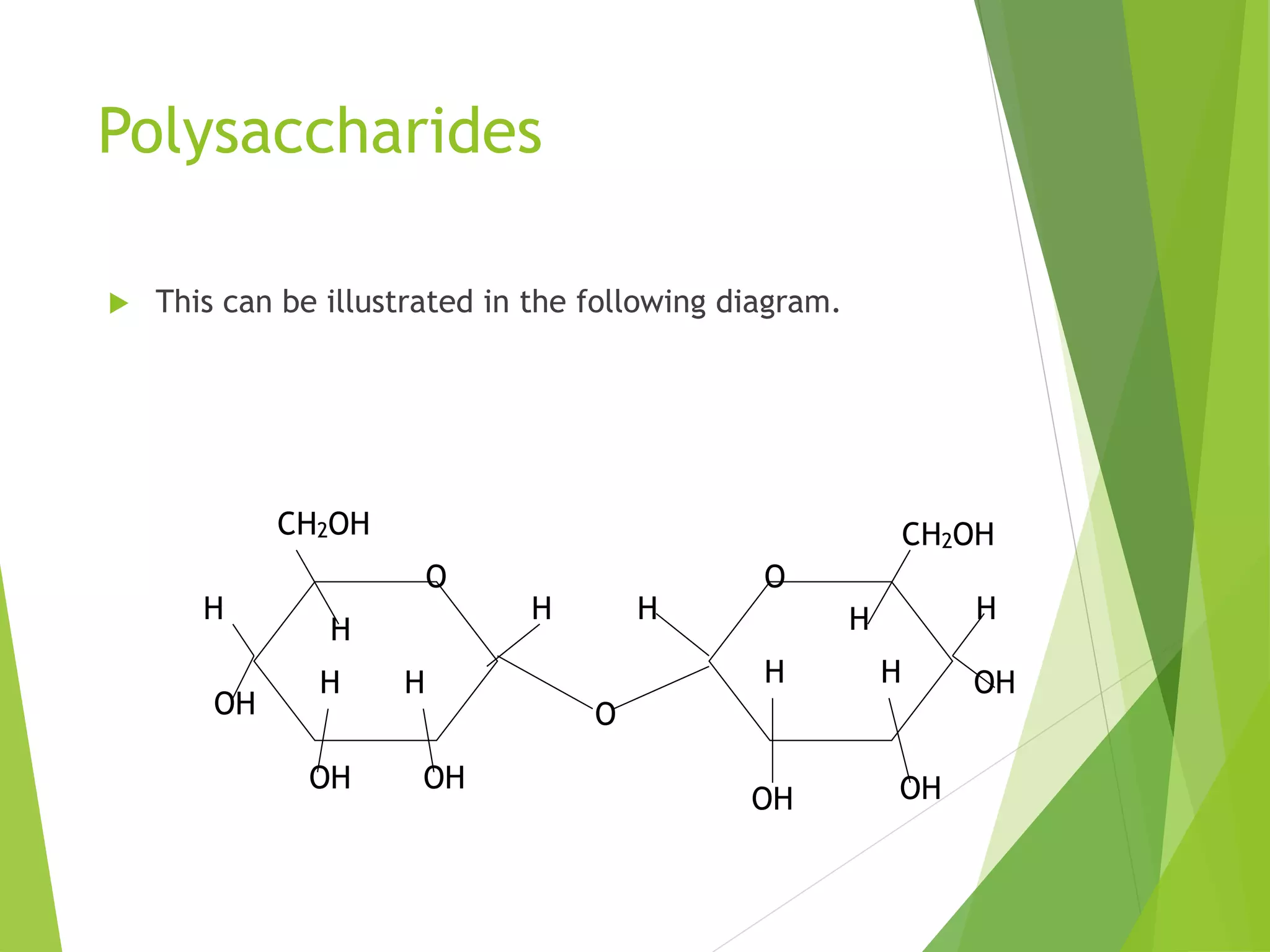
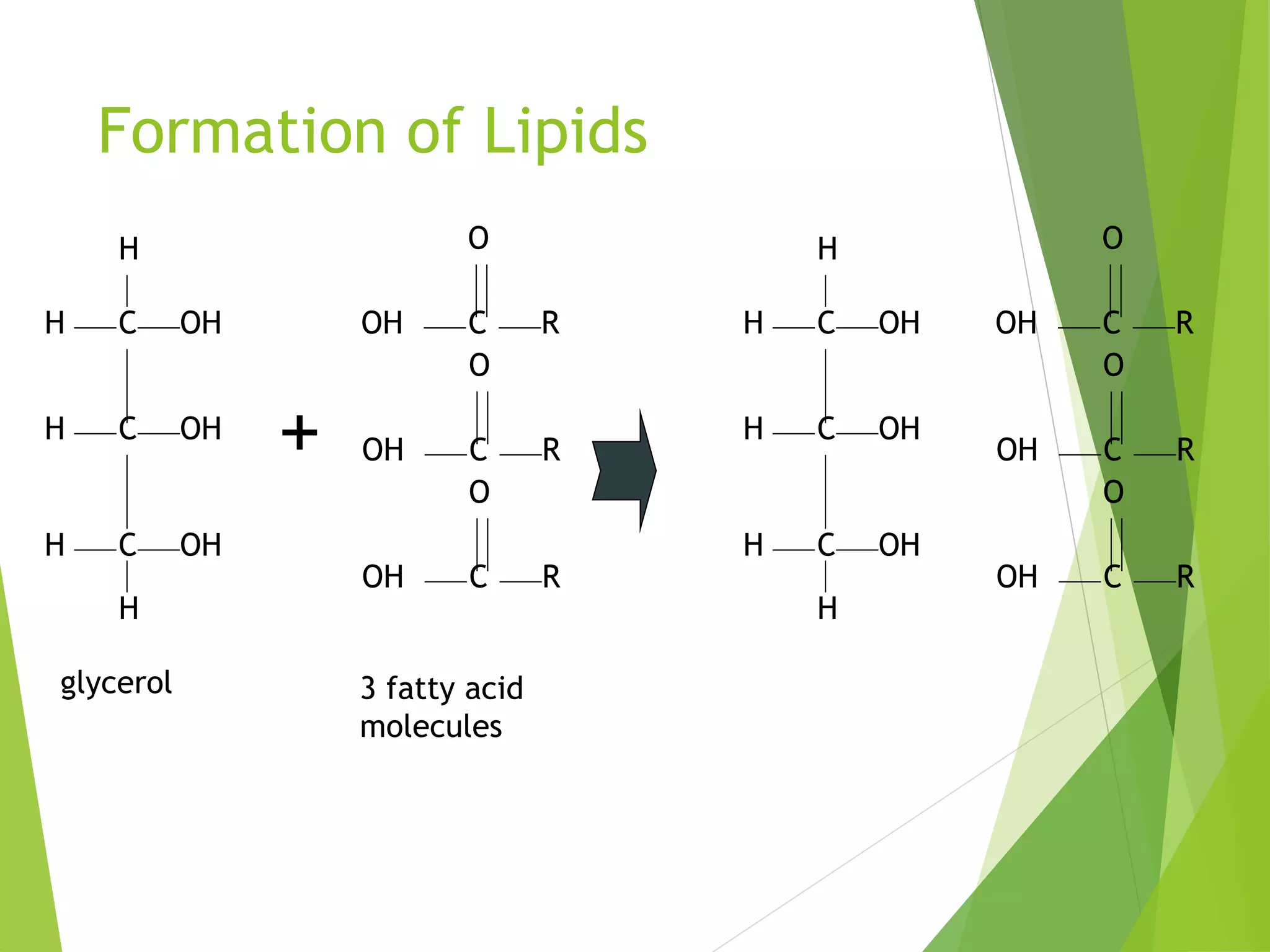
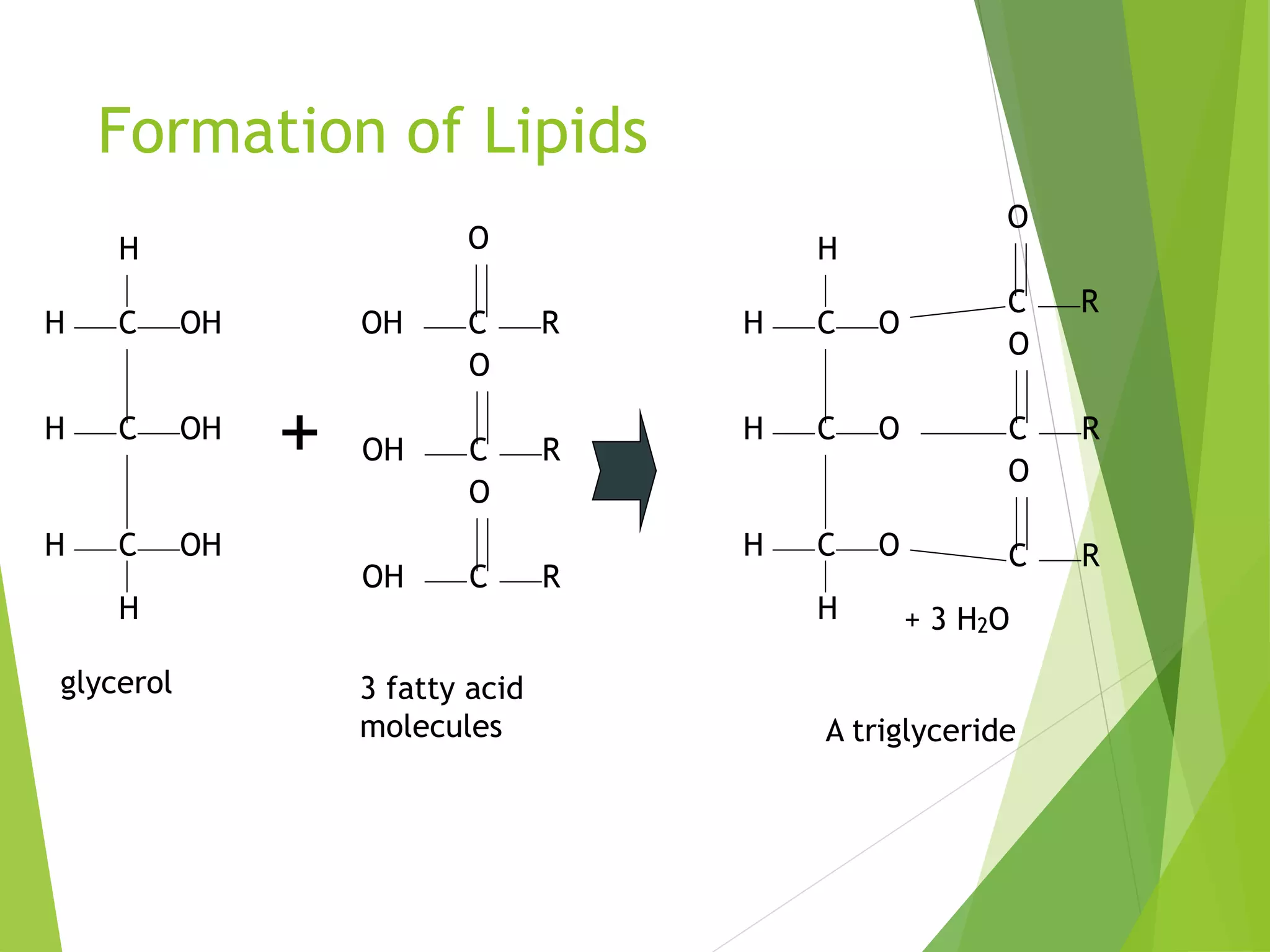
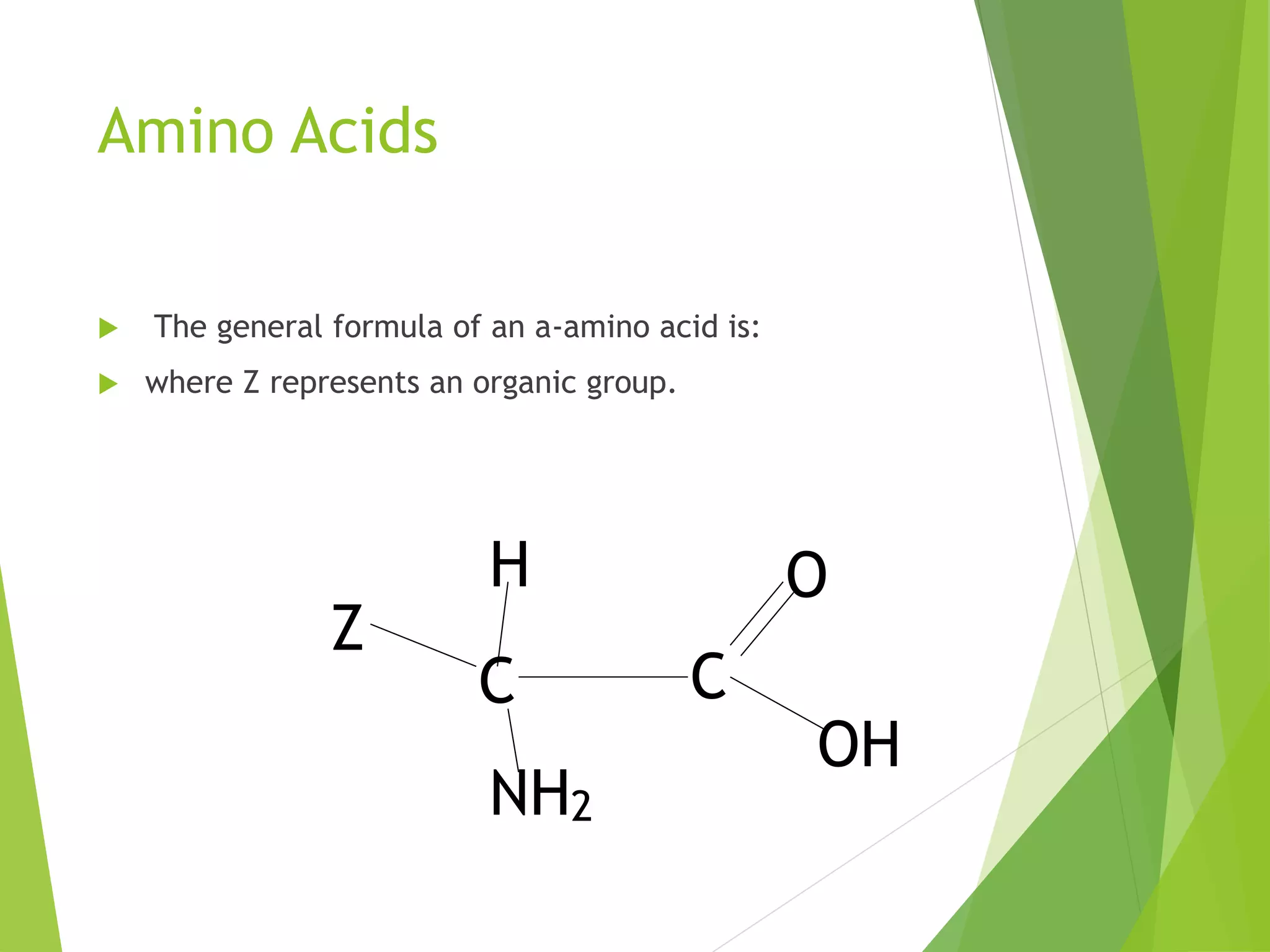
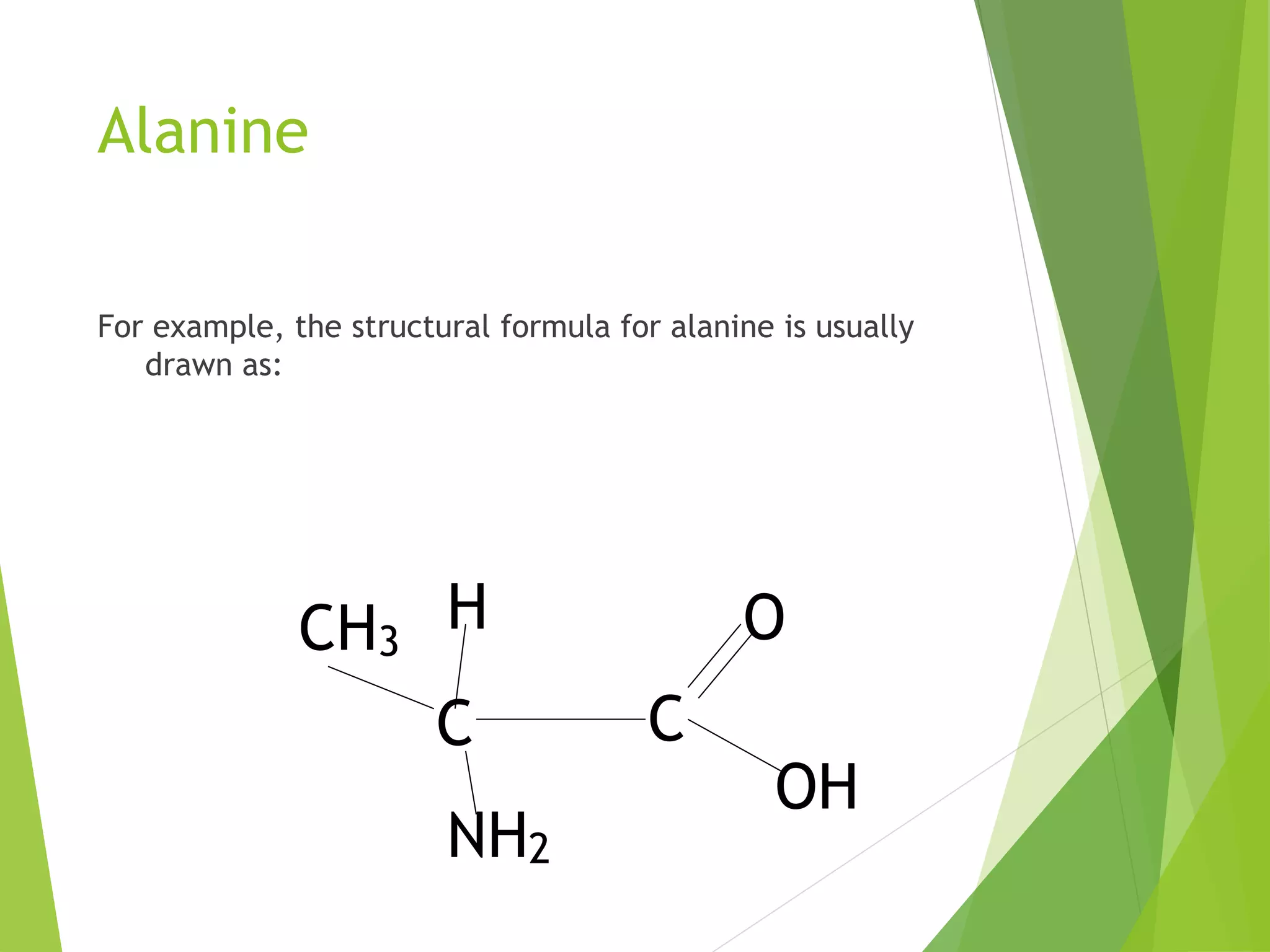
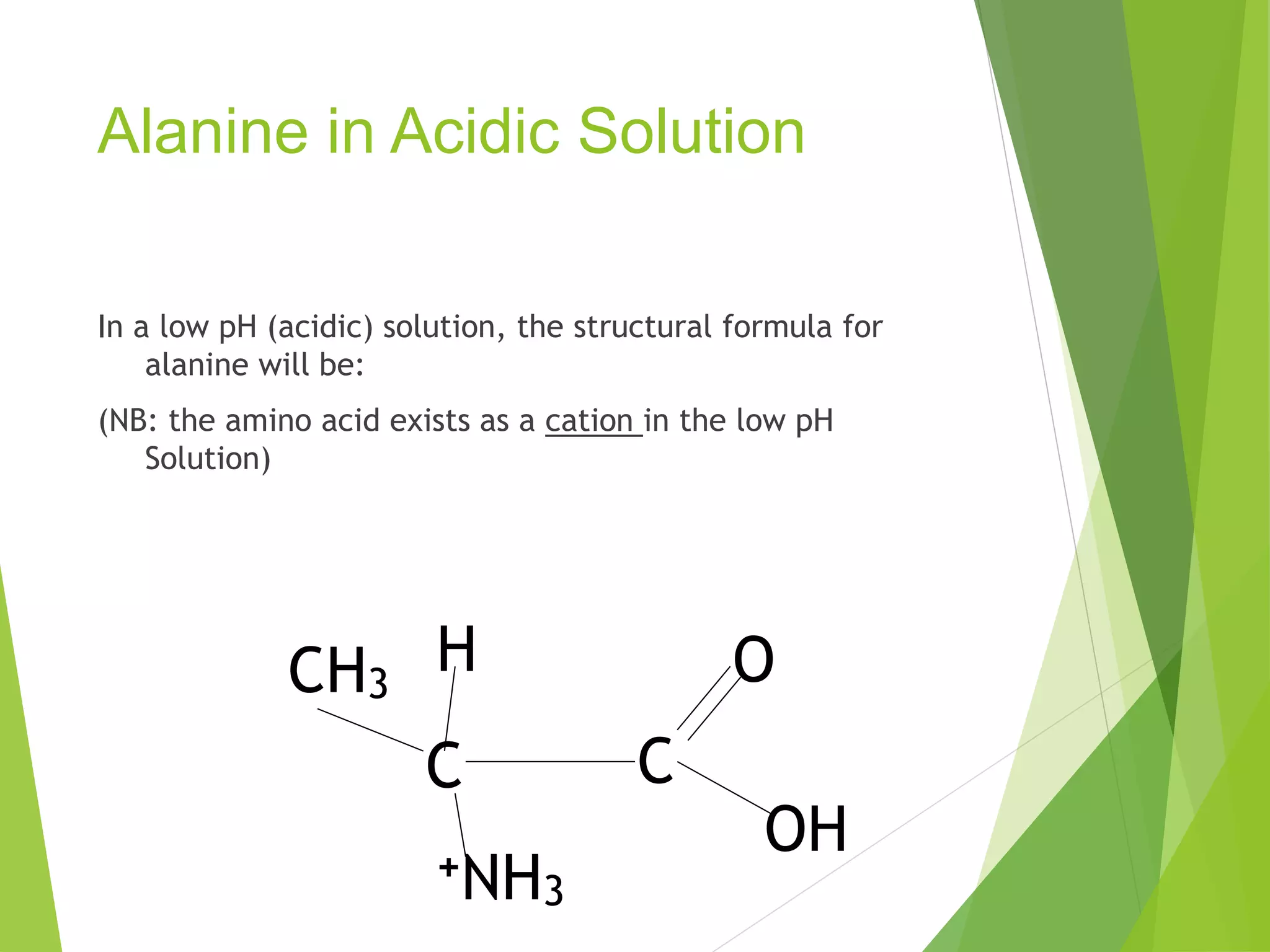
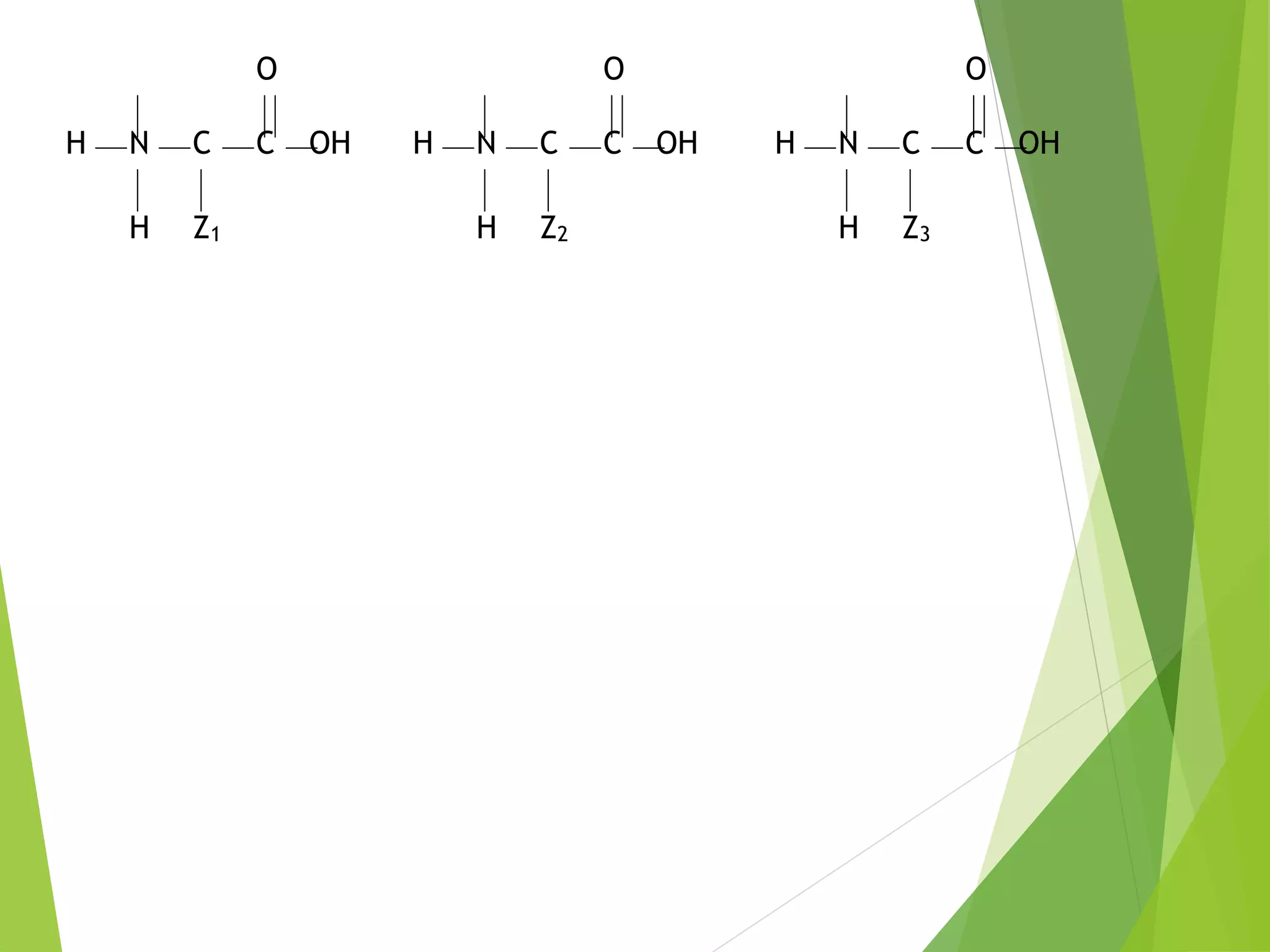
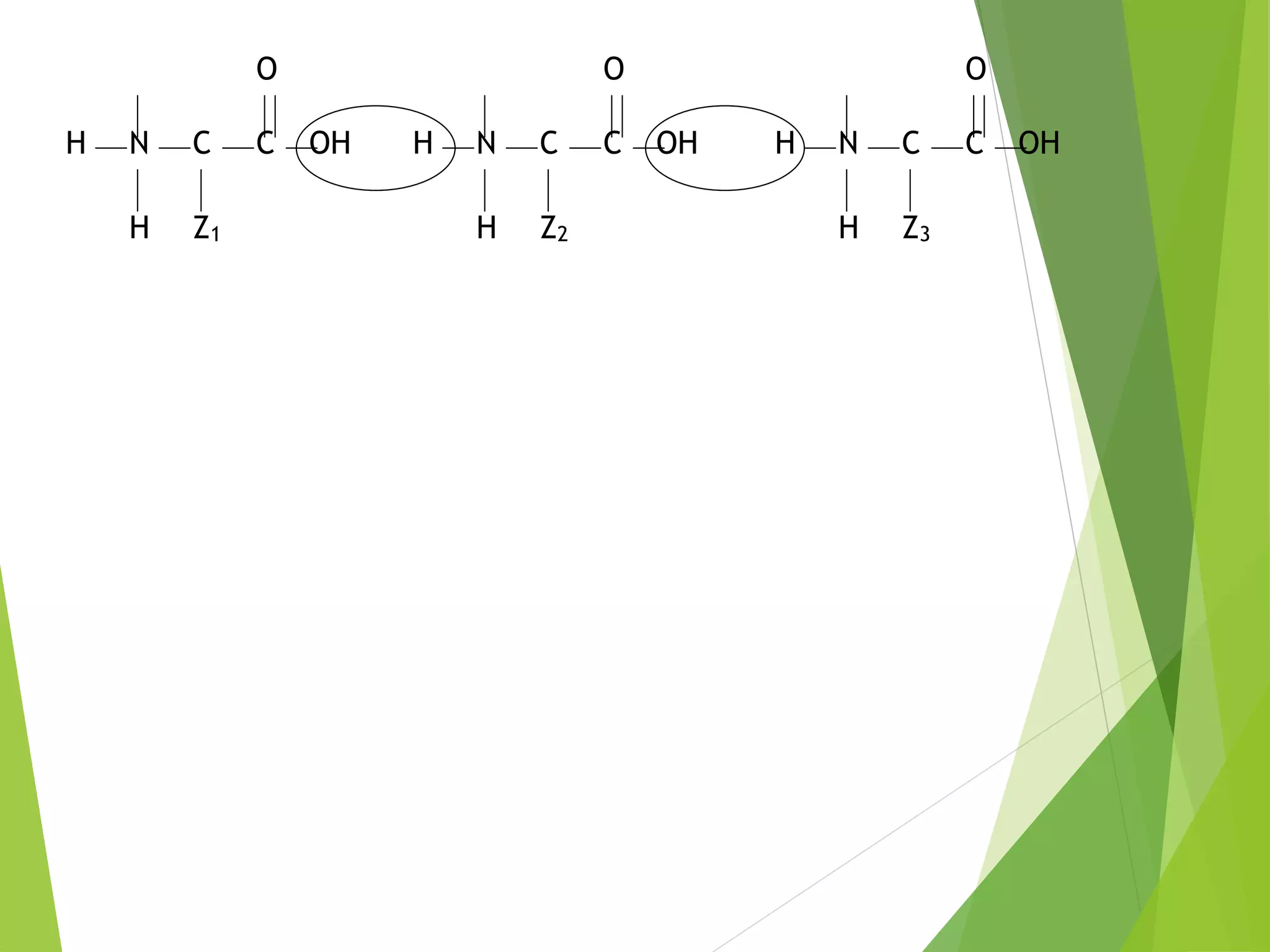
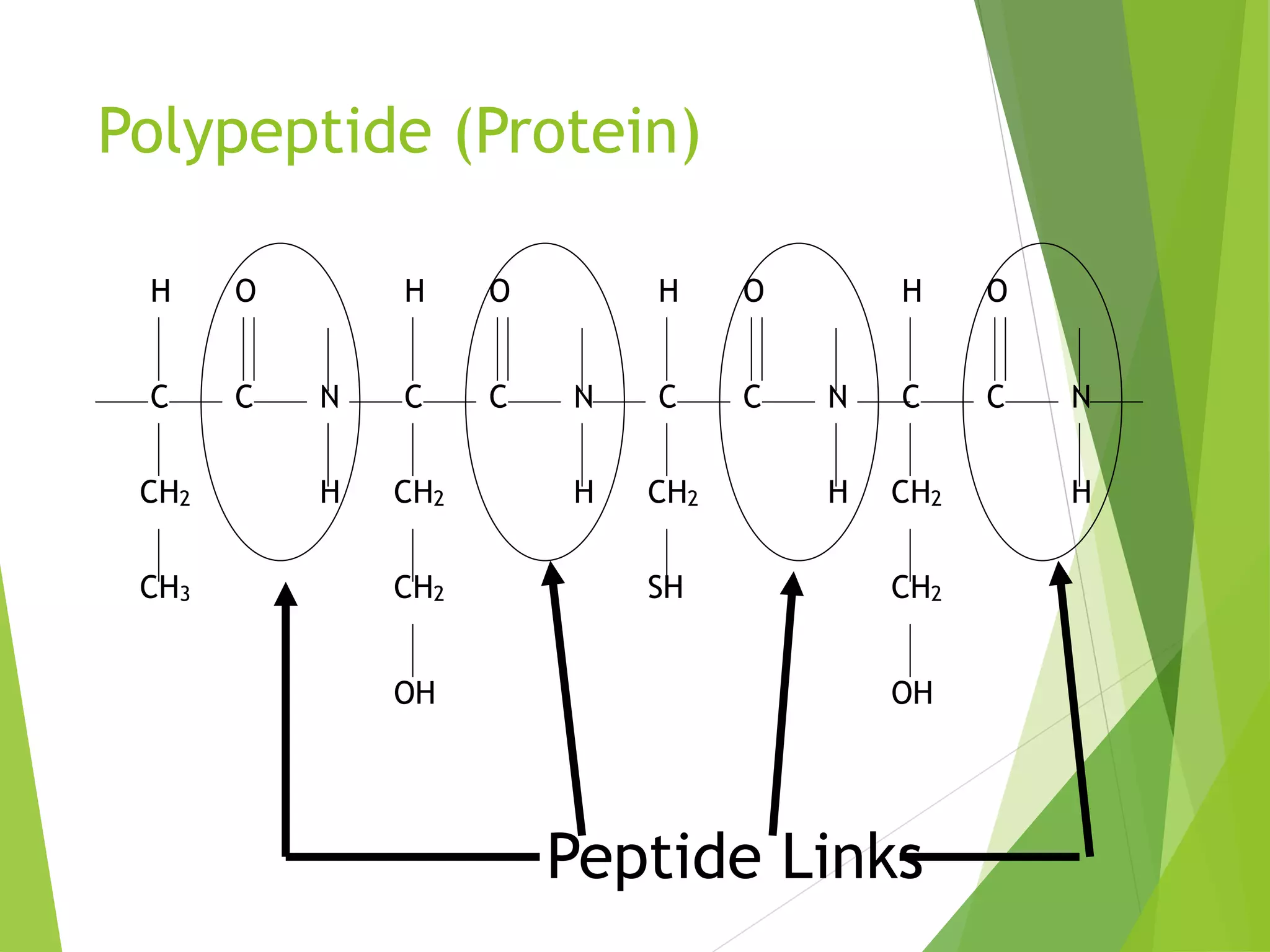
The document discusses the six major nutrients found in food - carbohydrates, lipids, proteins, mineral salts, vitamins, and water. It provides details on the structure and function of each nutrient, as well as typical food sources. The document also describes the human digestive system and the chemical processes that break down digested food, including the hydrolysis of carbohydrates, fats, and proteins into smaller molecules that can be absorbed.