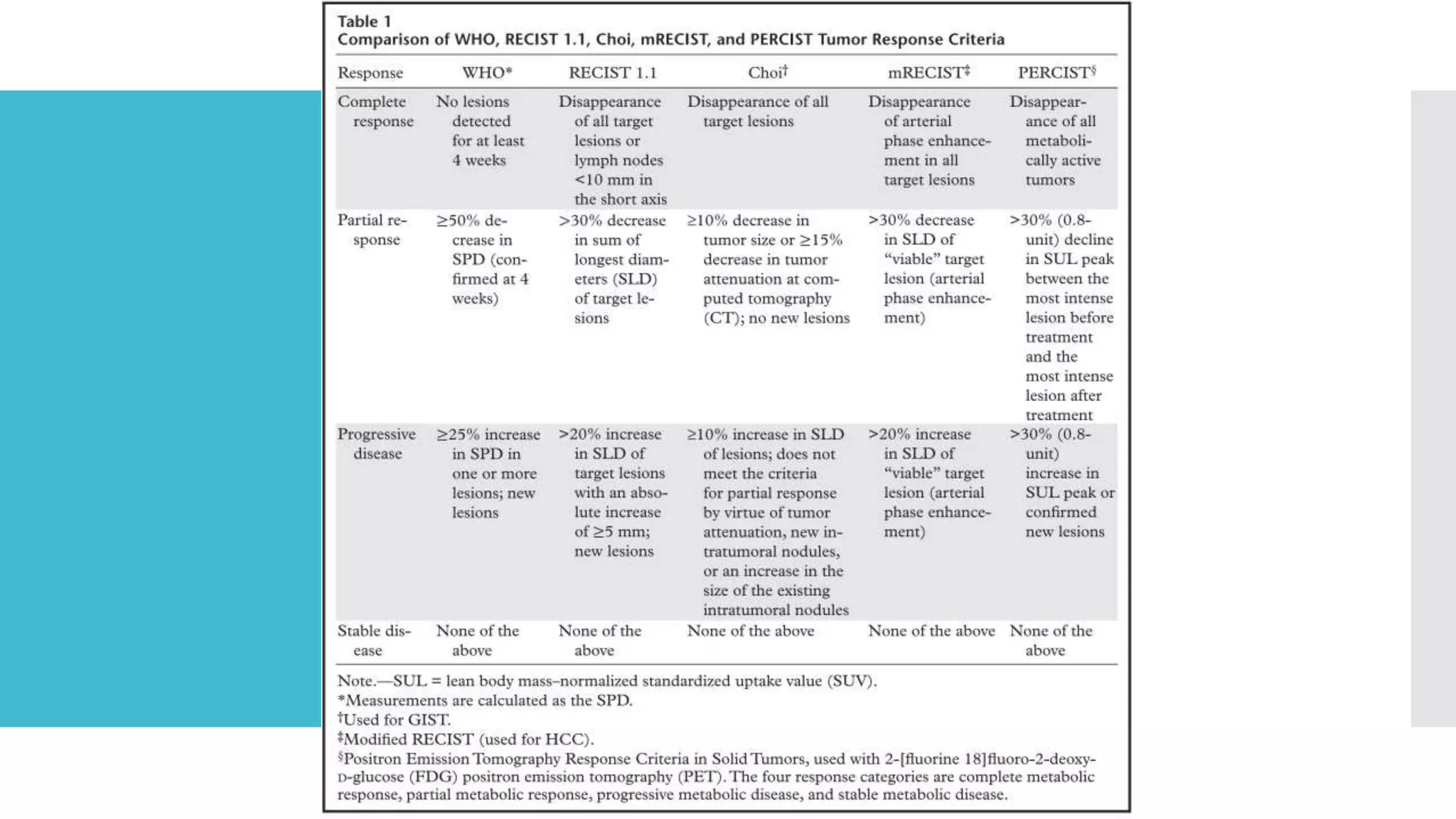
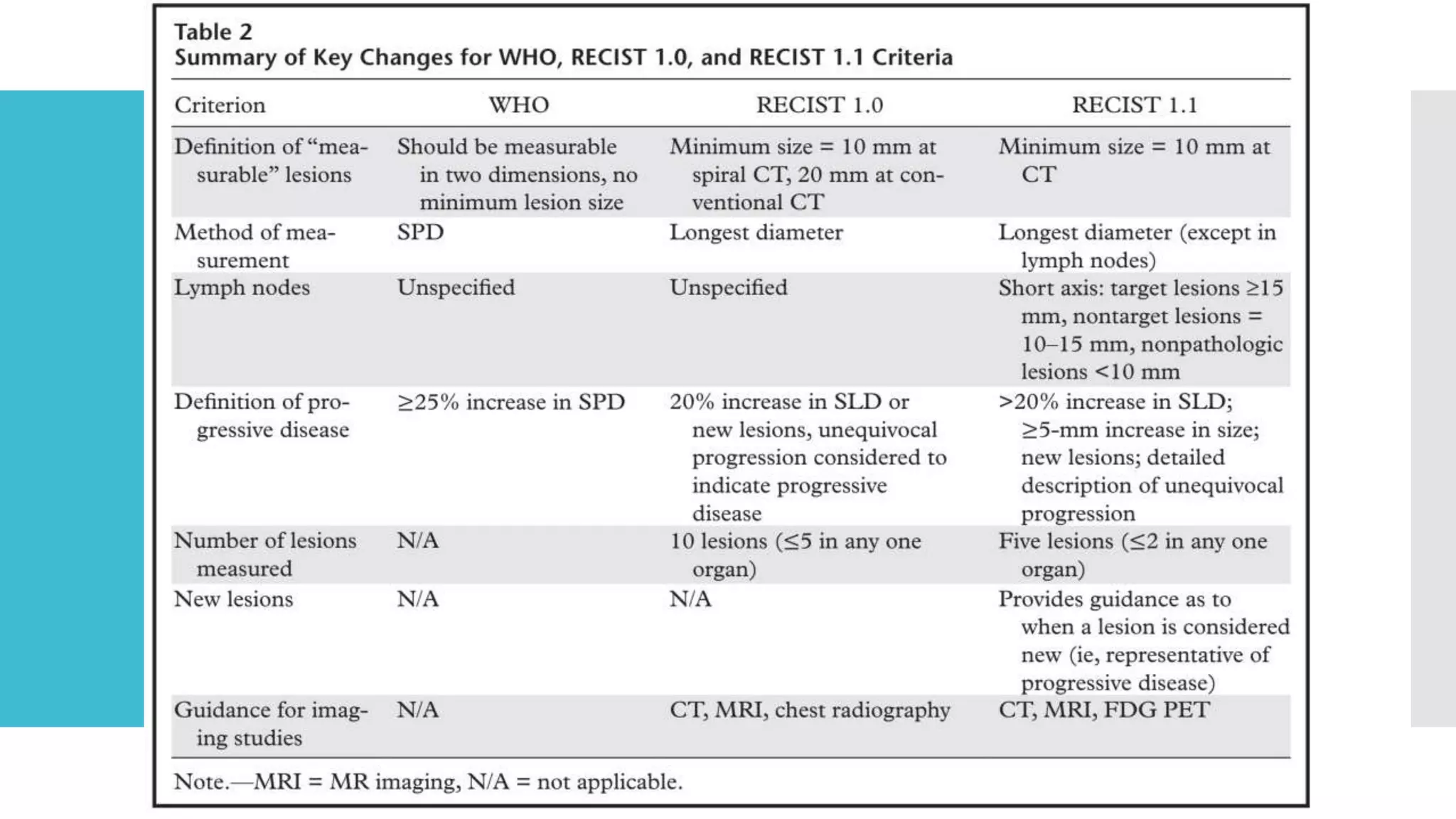
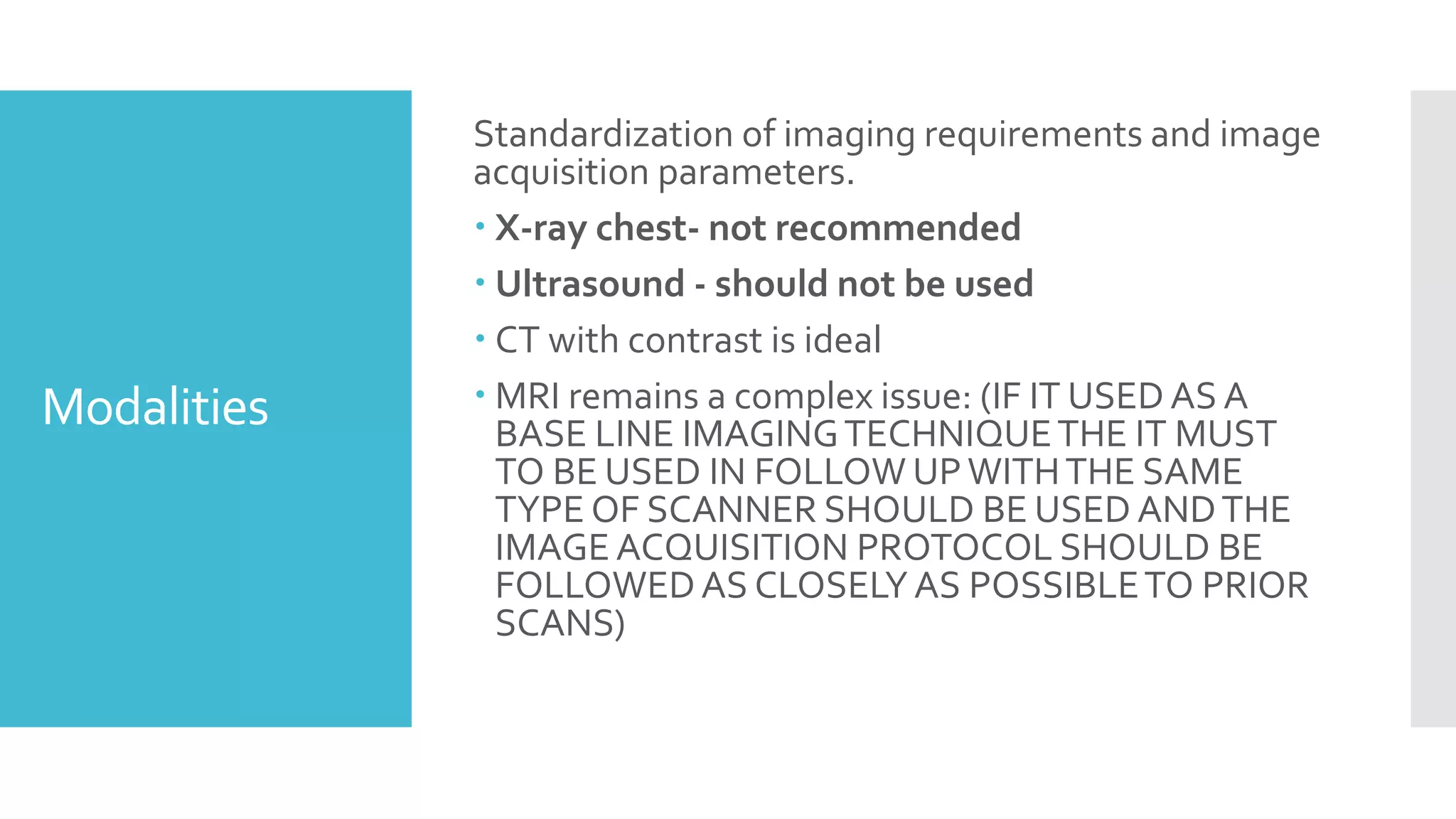
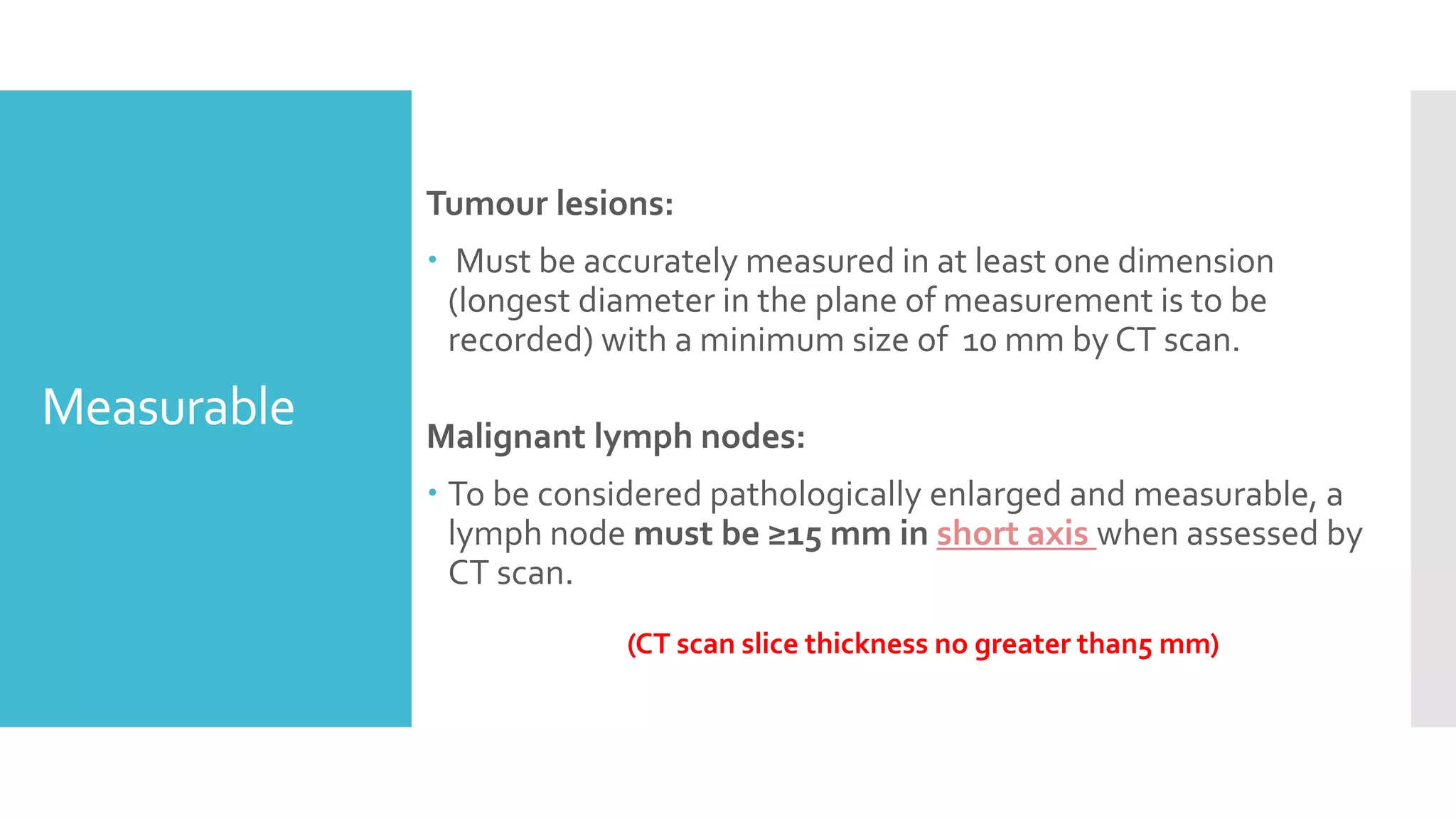
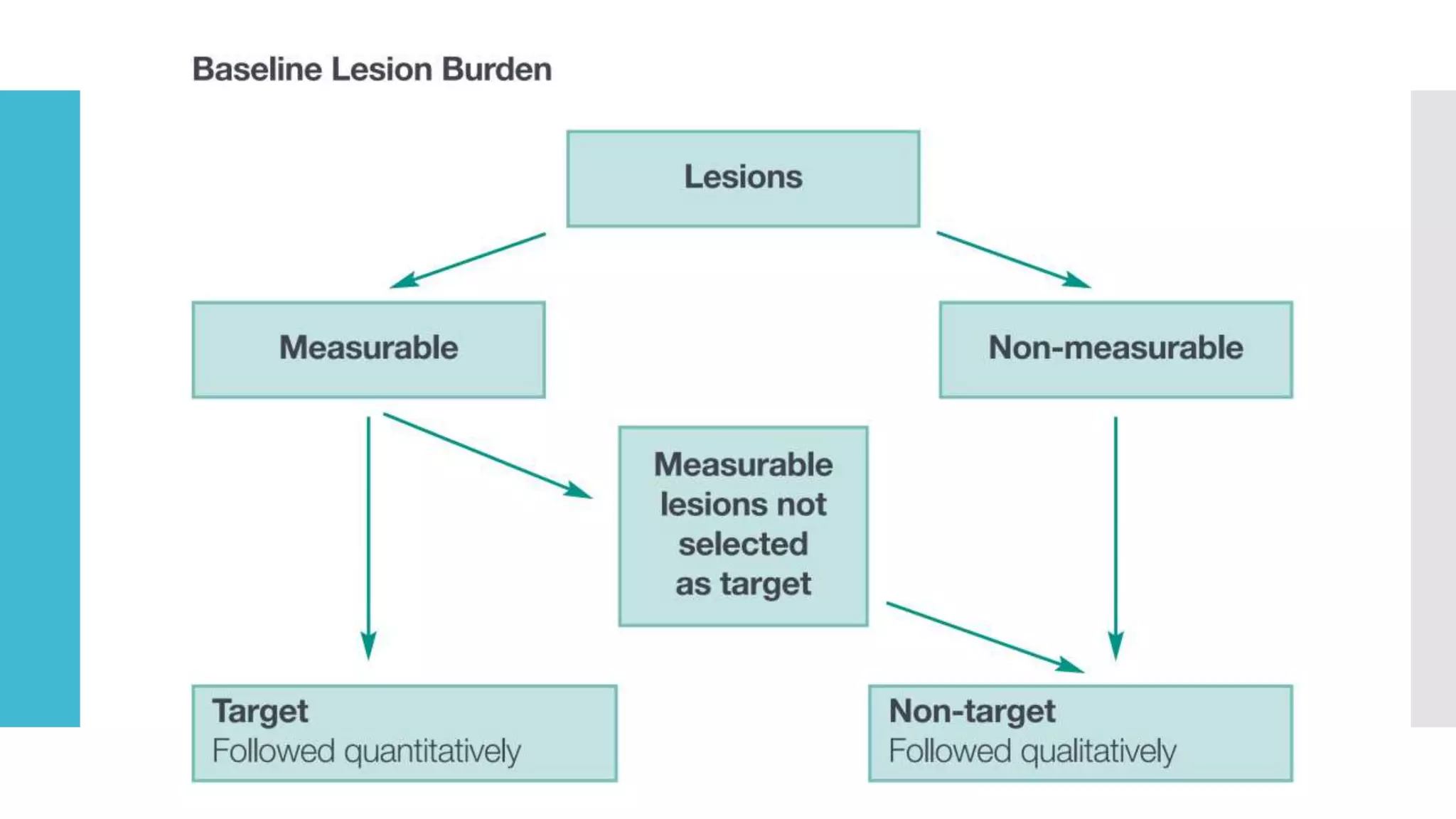
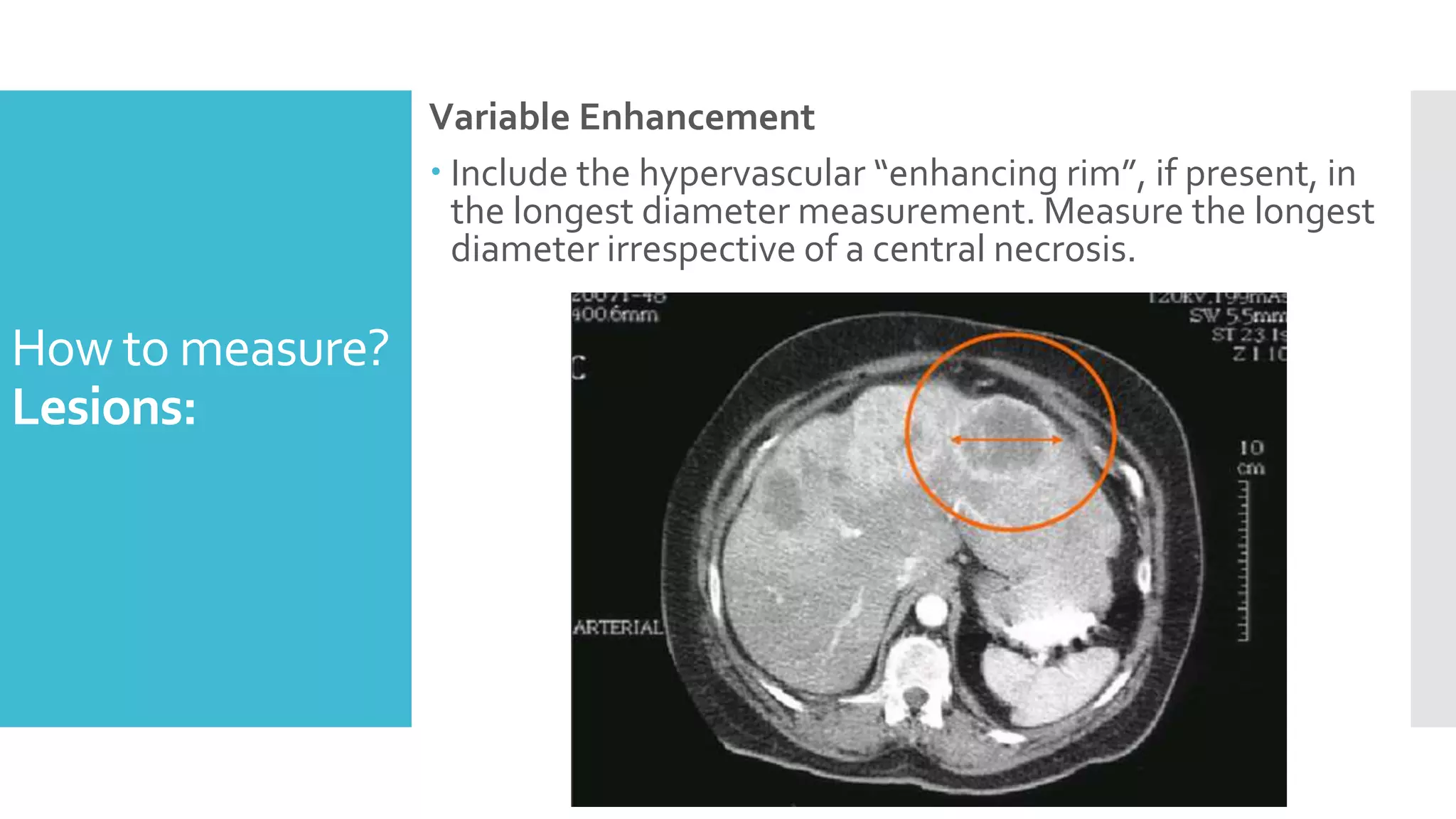
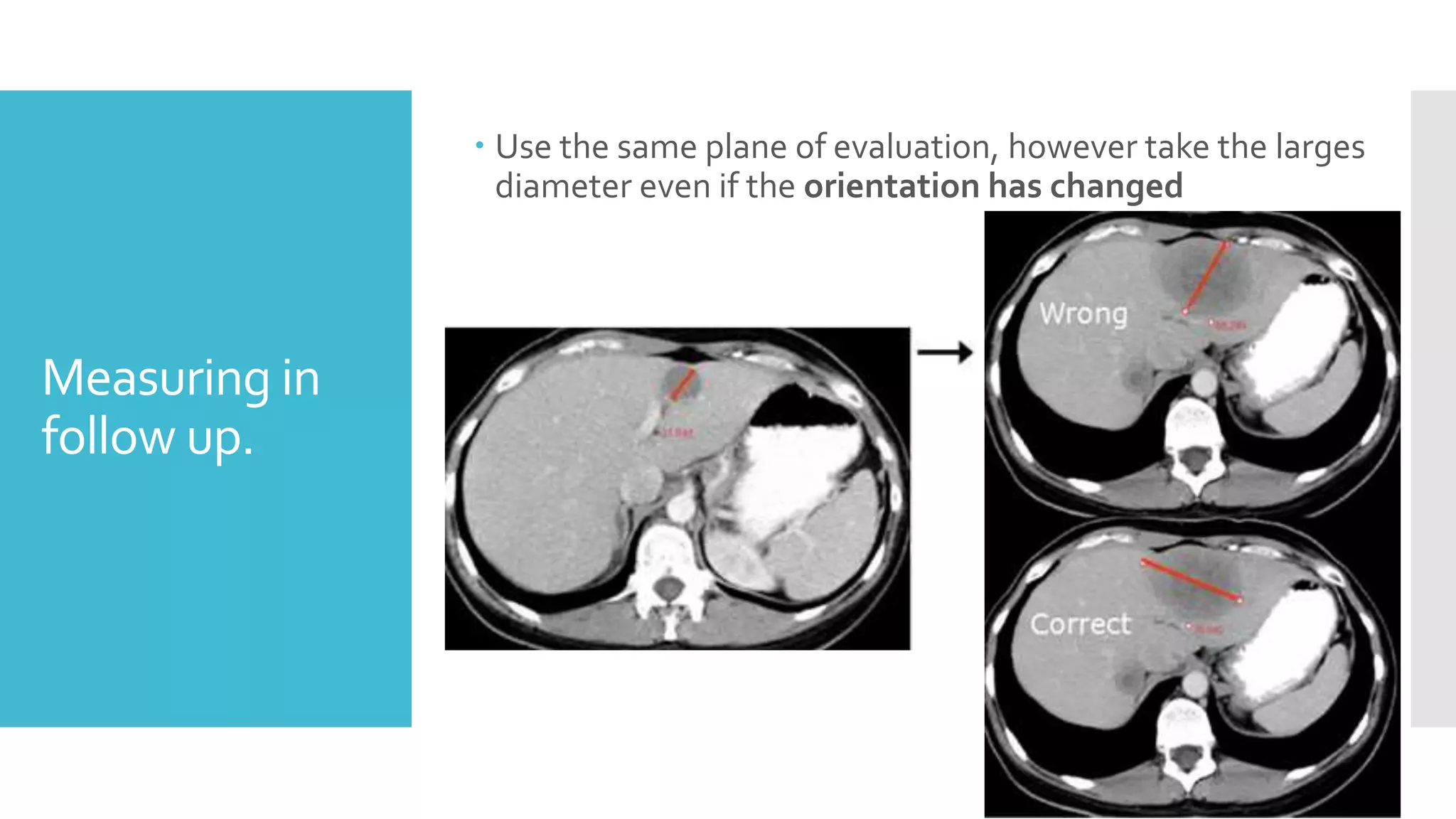
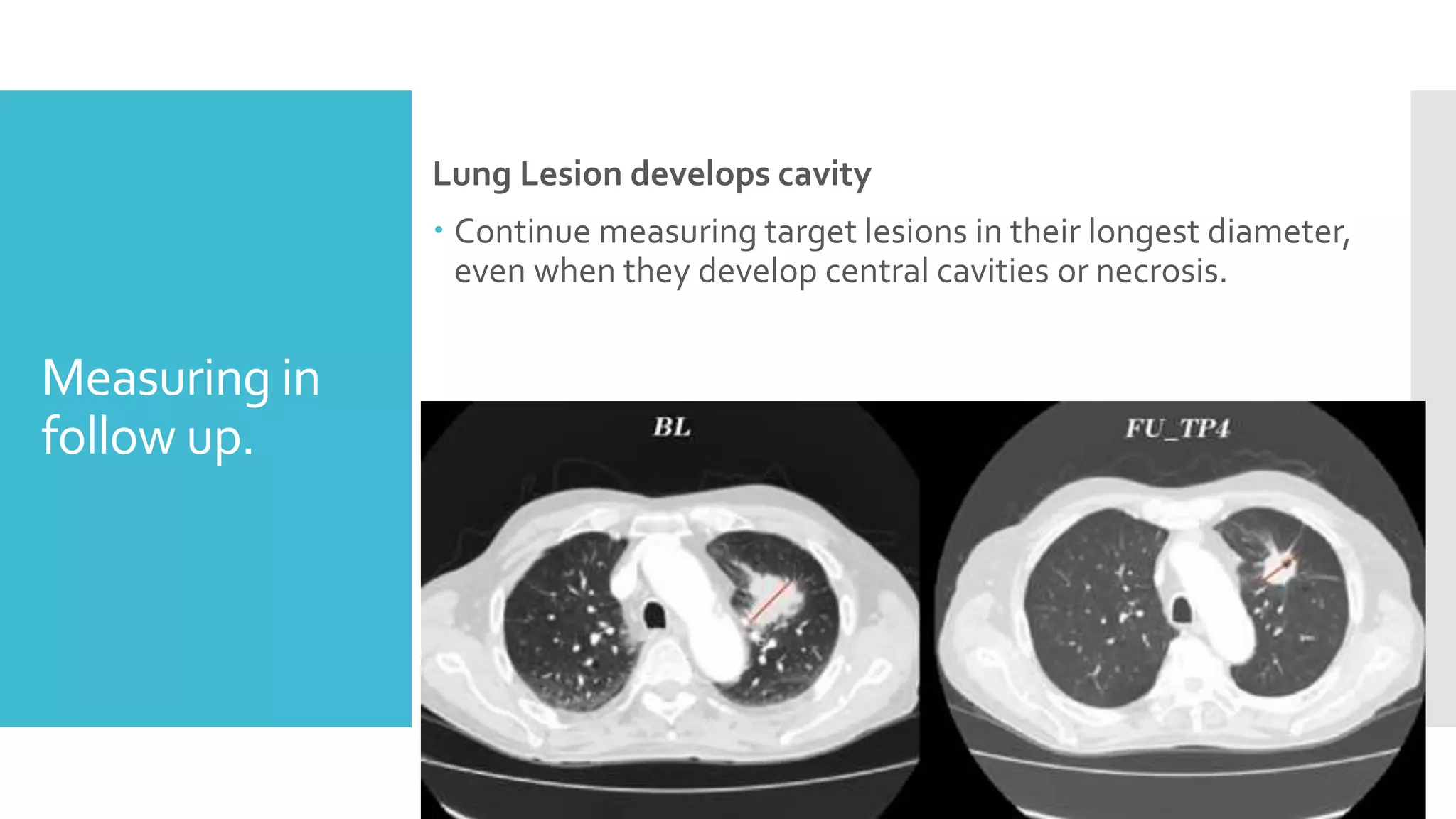
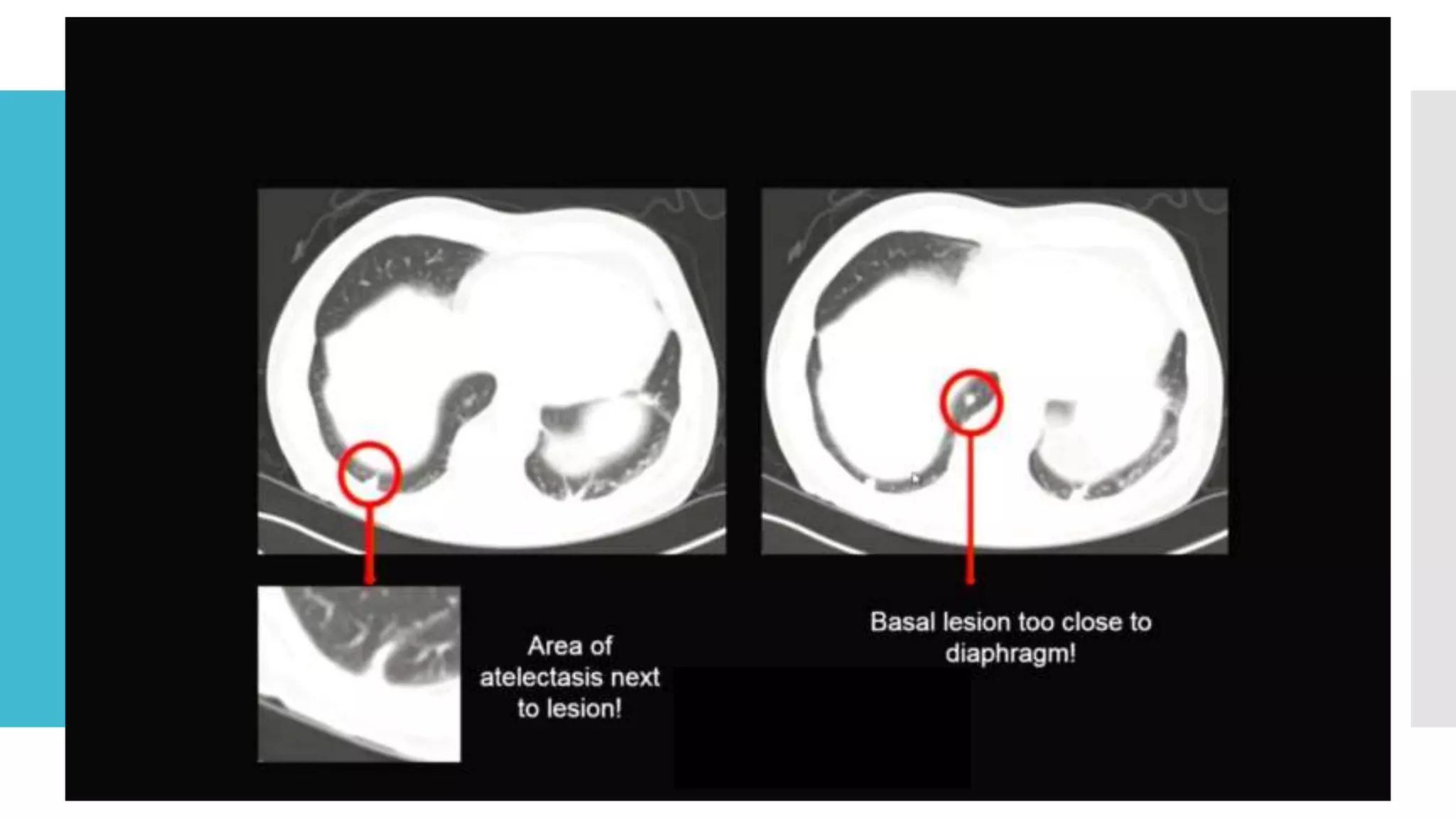
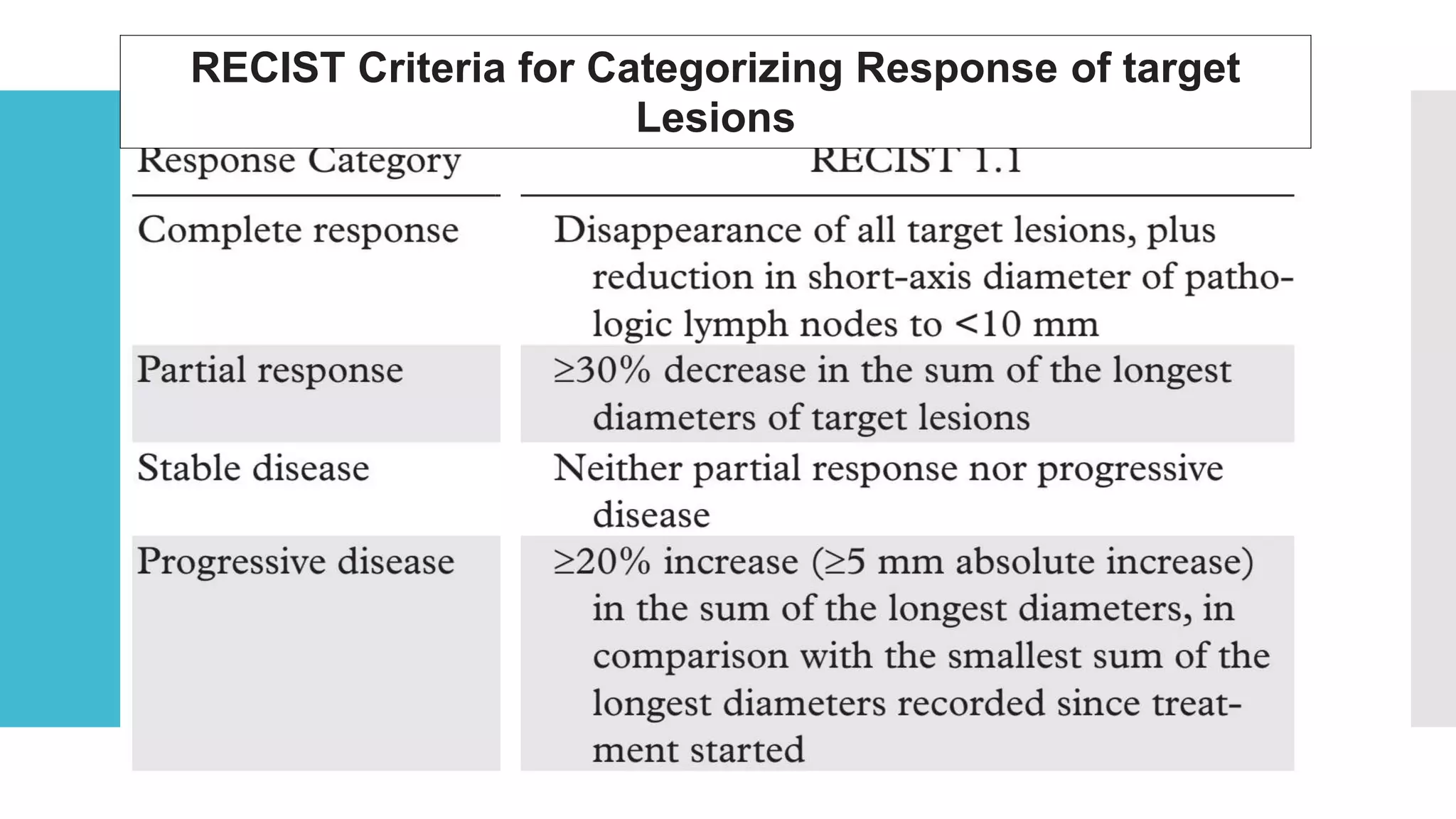
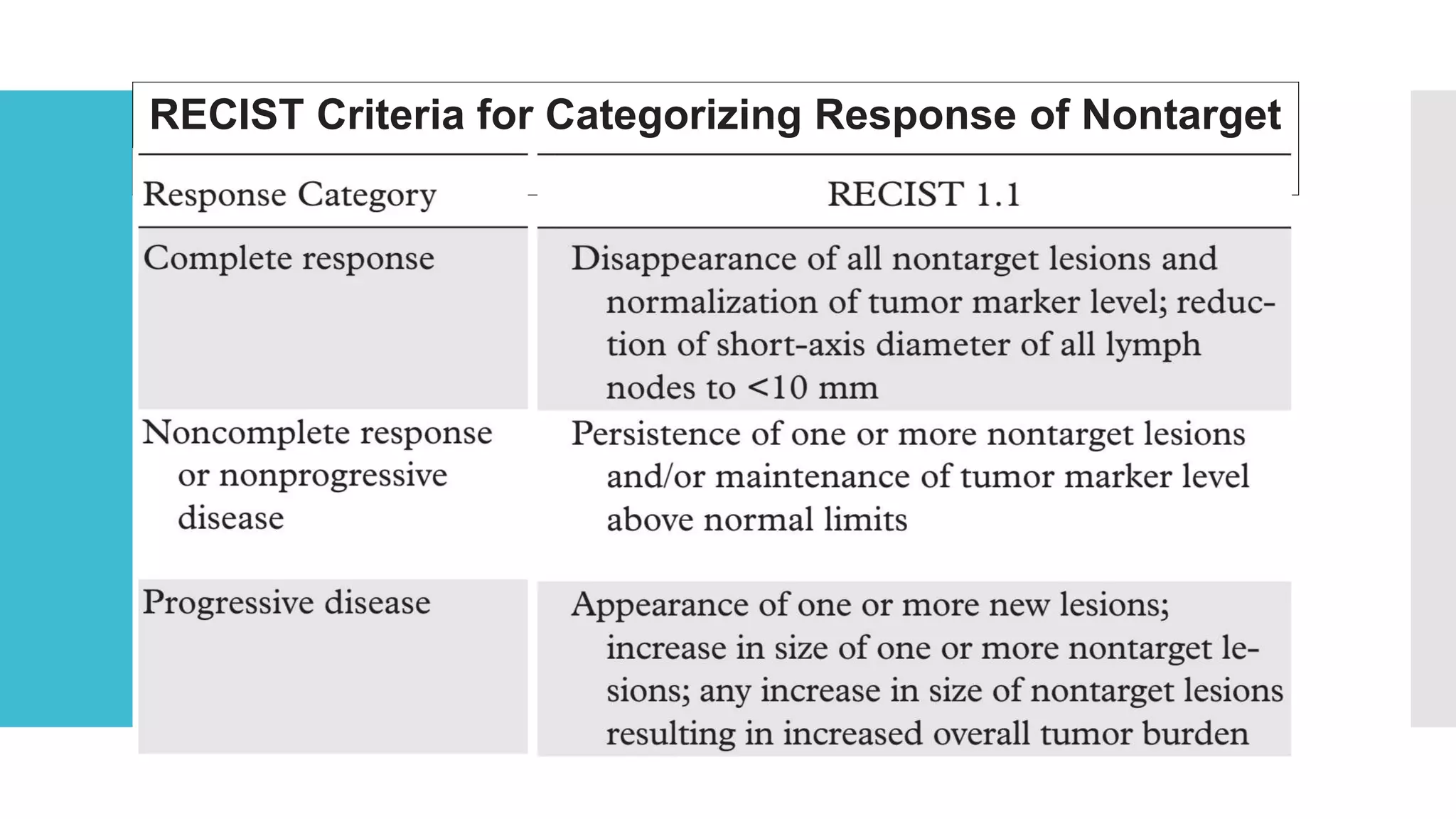
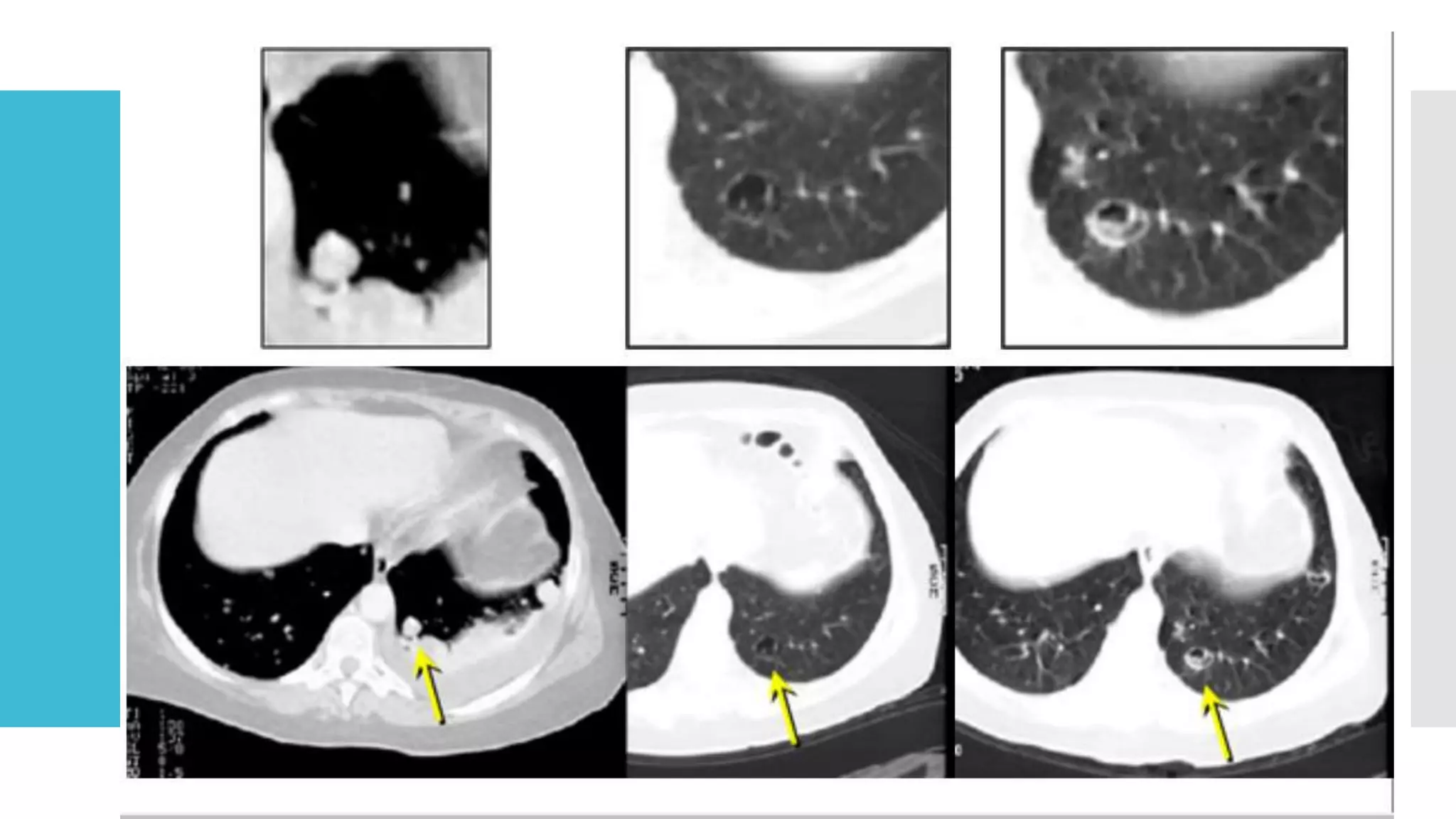
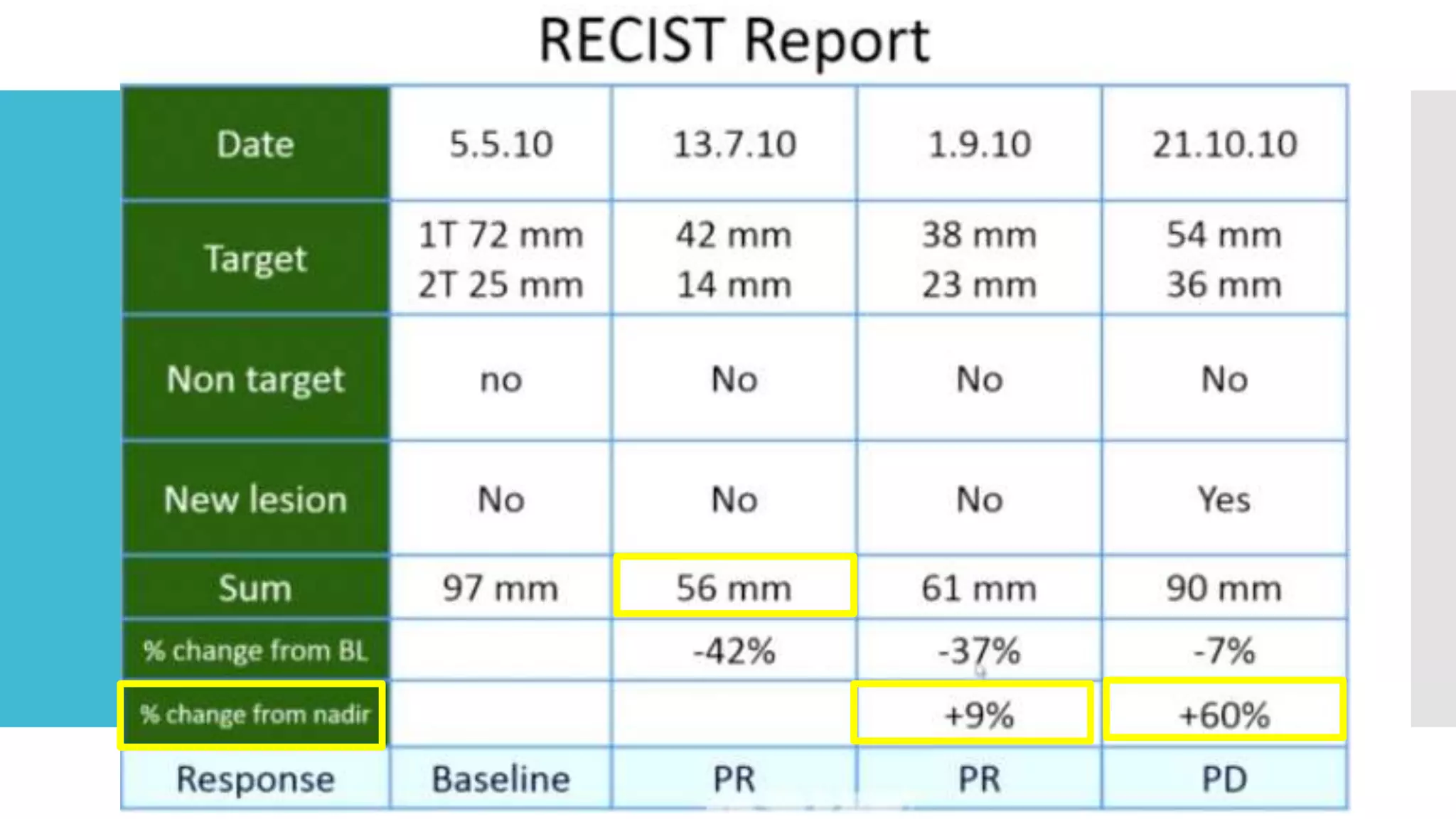
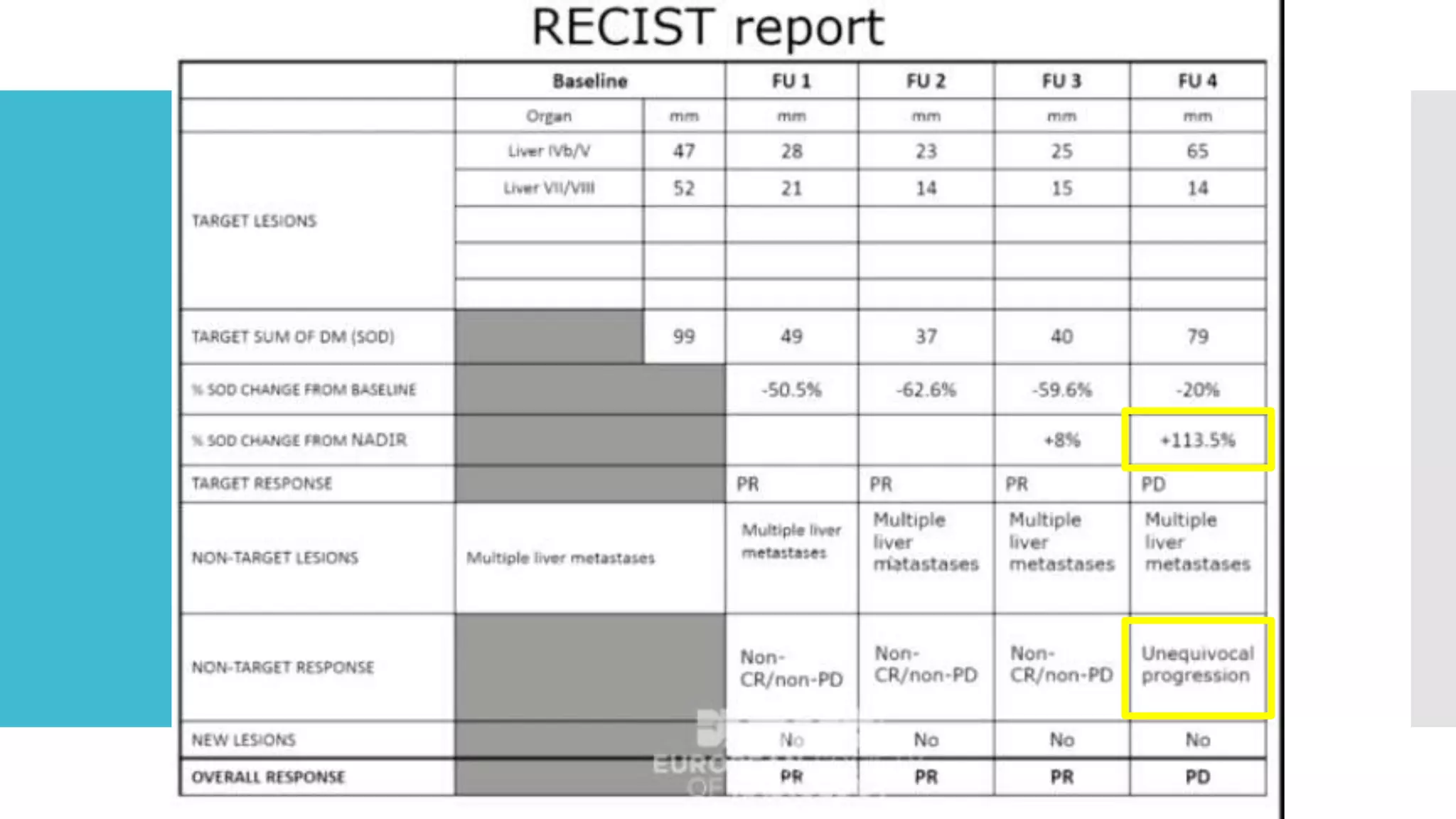
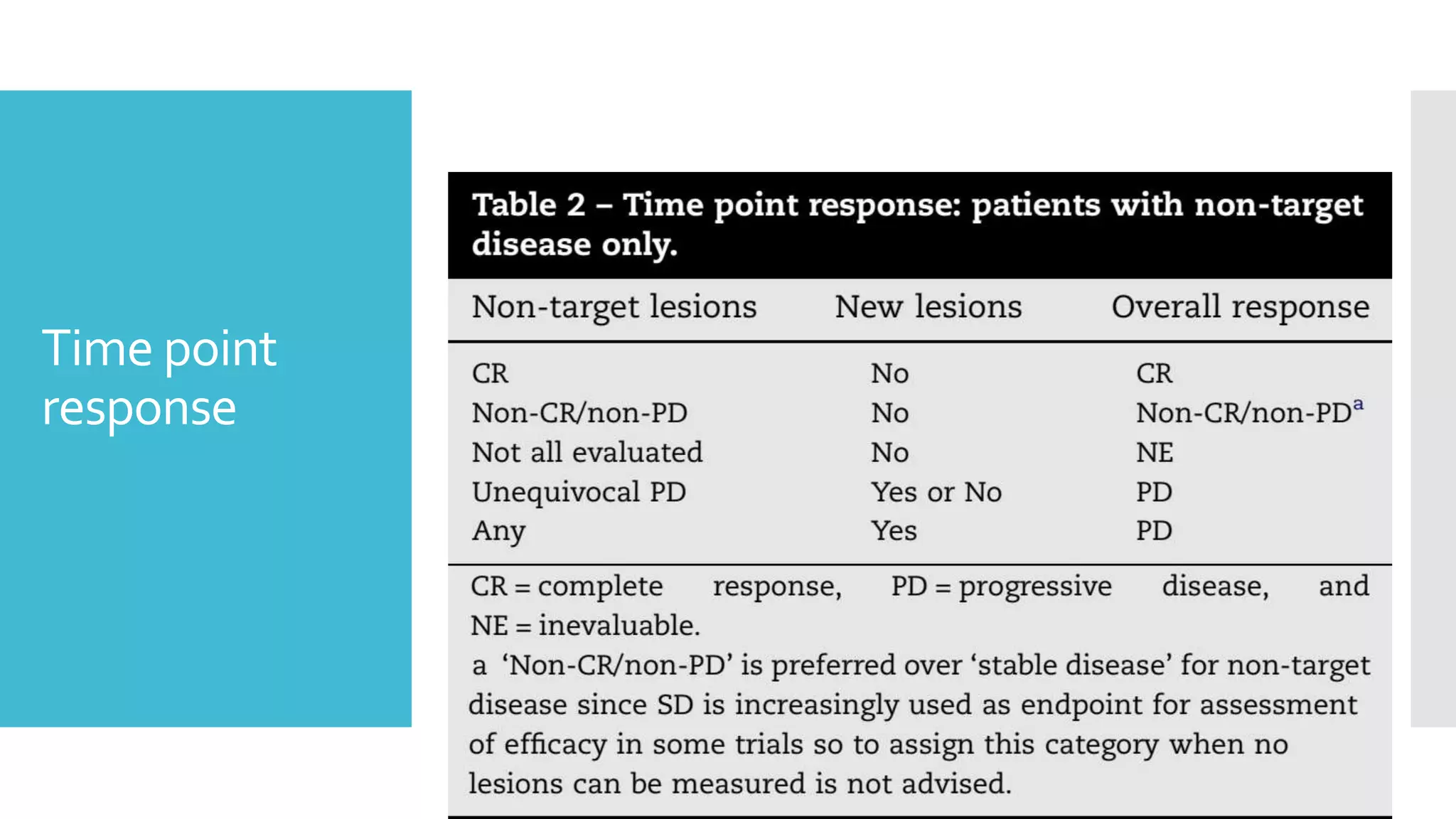
RECIST provides standardized criteria for evaluating tumor response to treatment in clinical trials. It defines criteria for complete response, partial response, stable disease, and progressive disease based on tumor measurements. Tumors must be accurately measured at baseline using CT or MRI. Target lesions are up to 5 measurable lesions selected for their ability to be reproducibly measured. Non-target lesions including small lesions and lymph nodes are also recorded. Tumor measurements are compared between scans to determine the patient's response according to RECIST criteria. The appearance of new lesions indicates disease progression.