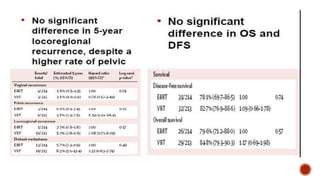
1) The PORTEC-1 and PORTEC-2 trials compared pelvic radiotherapy to no additional treatment or vaginal brachytherapy for patients with endometrial carcinoma. PORTEC-1 found pelvic radiotherapy reduced vaginal recurrence while PORTEC-2 found vaginal brachytherapy achieved excellent vaginal control with fewer side effects compared to pelvic radiotherapy.
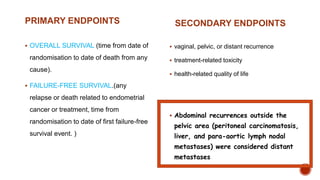
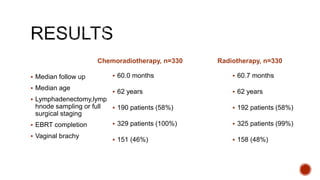
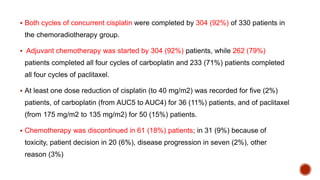
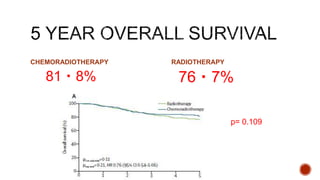
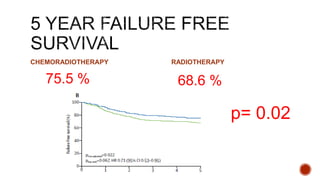
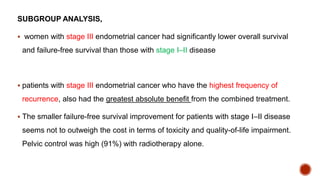
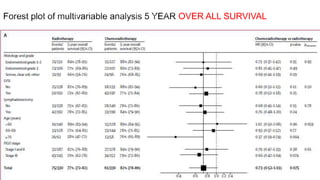
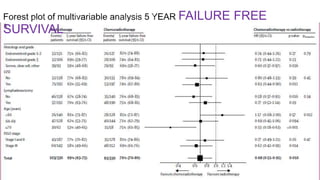
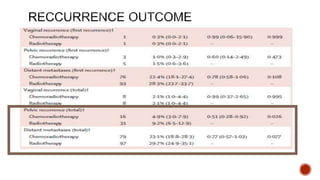
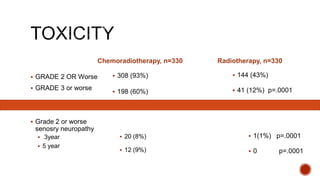
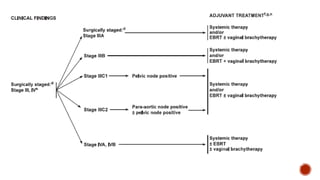
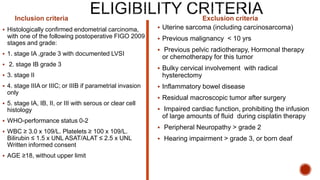
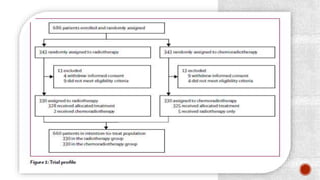
2) The PORTEC-3 trial randomized 686 patients with high risk endometrial cancer to chemoradiotherapy or radiotherapy alone. It found chemoradiotherapy improved failure-free survival compared to radiotherapy alone, especially for stage III patients, but with increased toxicity.
3)
















![ Given in both treatment group
Total dose 48.6 Gy 1.8 Gy fractions , 5 days a week
Proximal vagina,Parametrial tissue
Internal, external, and common iliac node upto L5- S1(with a margin of ≥2 cm above the highest
involved lymph node).
In case of cervical involvement (glandular, stromal, or both), a brachytherapy boost was given to
the vaginal vault.
Brachytherapy dose was equivalent to 14 Gy in 2 Gy fractions (with recommended scheme of 10
Gy high-dose rate [HDR] in fractions of 5 Gy), specified at 5 mm from the vaginal vault surface.
start within 4–6 weeks of surgery, but no later than 8 weeks.](https://image.slidesharecdn.com/portec3-180331180716/85/Portec-3-20-320.jpg)