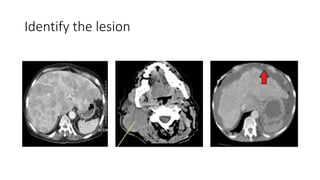
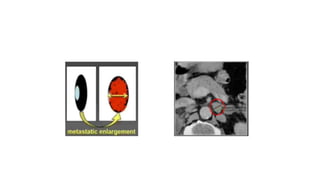
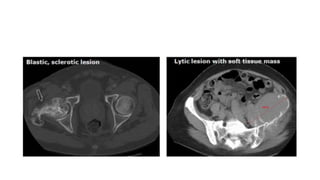
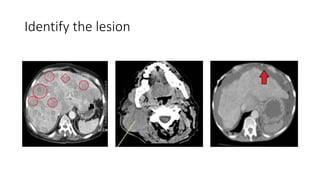
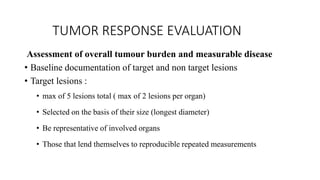
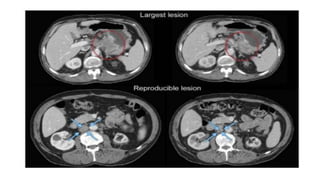
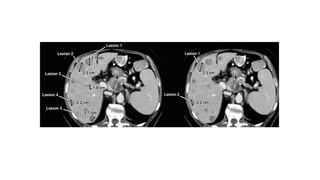
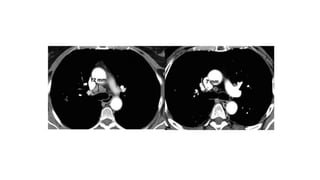
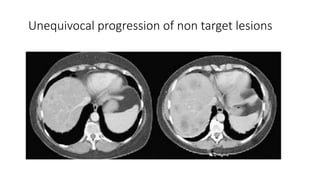
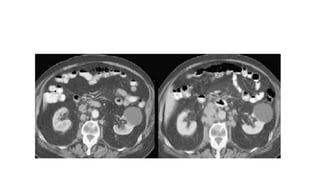
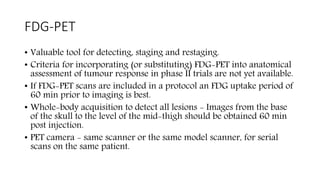
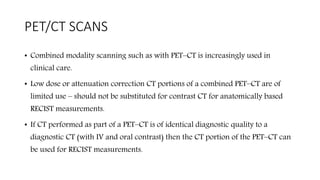
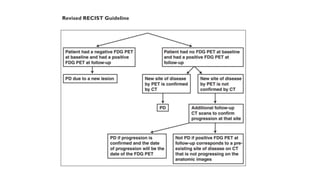
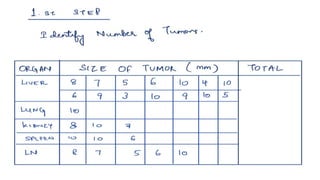
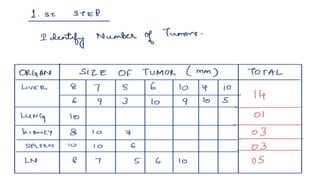
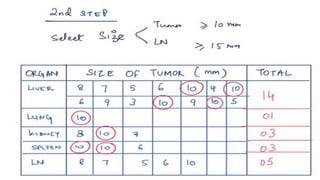
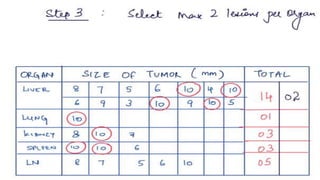
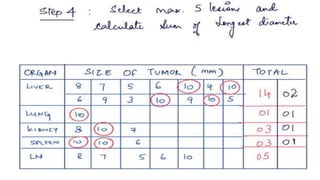
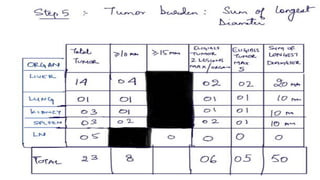
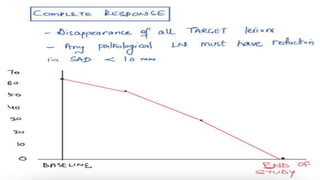
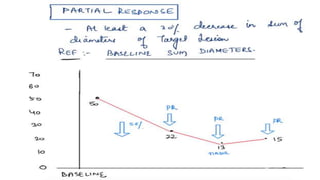
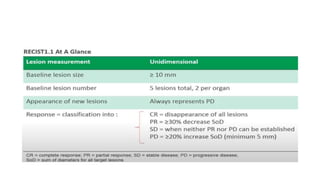
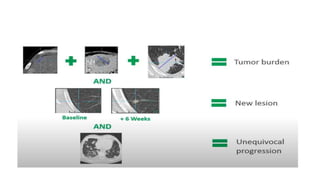
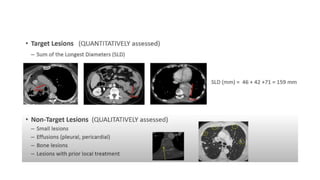
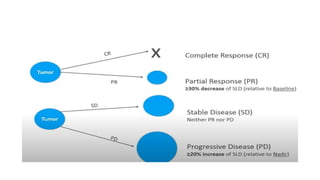
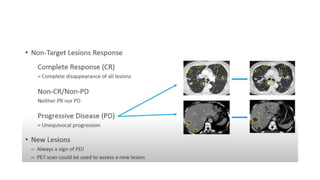
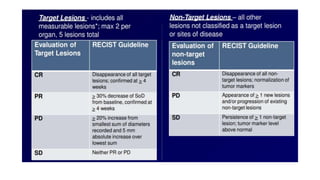
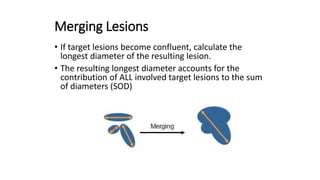
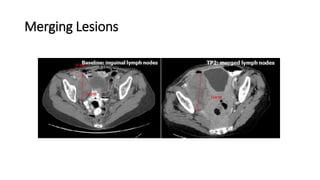
RECIST 1.1 provides standardized criteria for evaluating tumor response in cancer clinical trials. It was updated from RECIST 1.0 to improve standardization. Key changes include: clarifying how to measure and evaluate target and non-target lesions including lymph nodes; requiring confirmation of complete response; and clarifying the definition of progressive disease. RECIST evaluates tumor burden based on measuring the longest diameter of up to five target lesions with a maximum of two lesions per organ. Response categories include complete response, partial response, stable disease, and progressive disease.