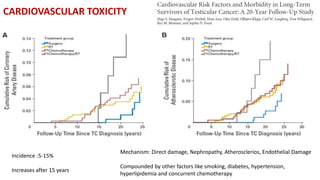
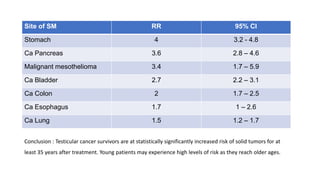
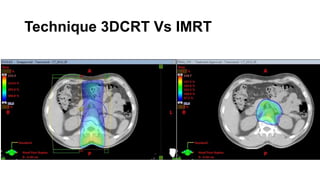
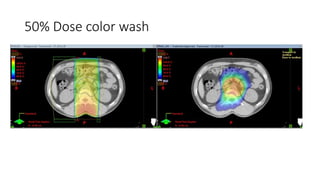
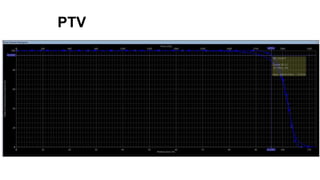
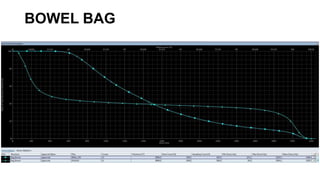
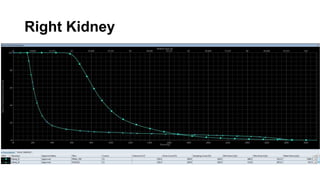
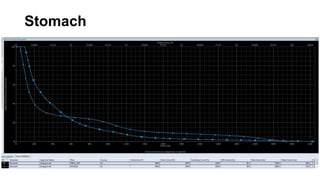
Radiotherapy for Seminoma
- Seminoma accounts for over 60% of testicular germ cell tumors with an incidence of 0.95 per 100,000.
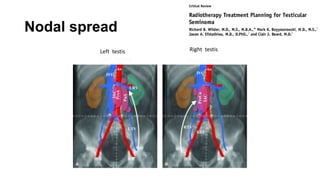
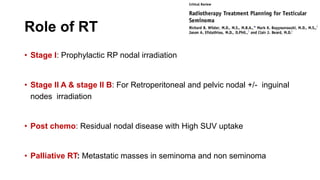
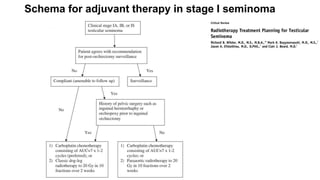
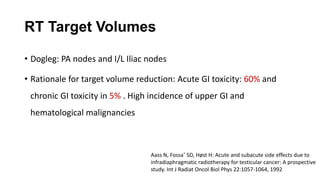
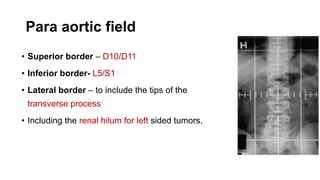
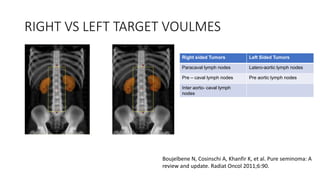
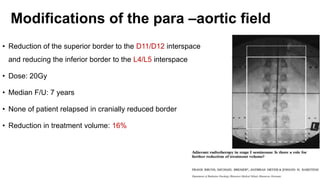
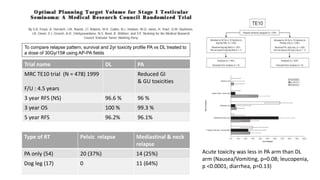
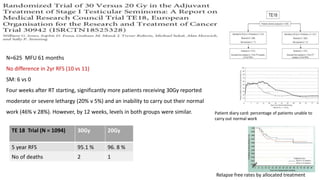
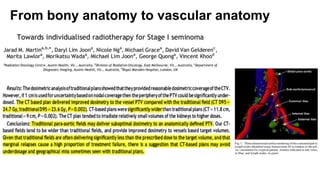
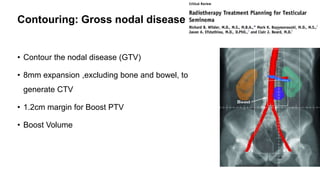
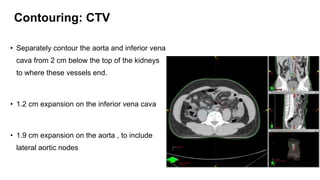
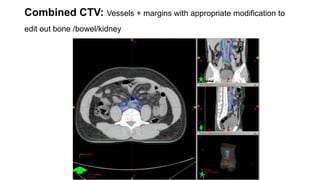
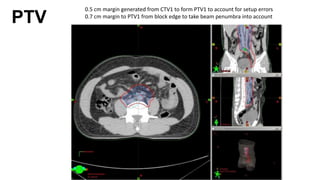
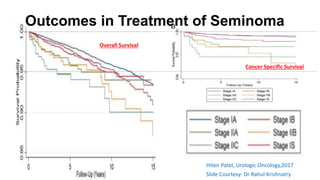
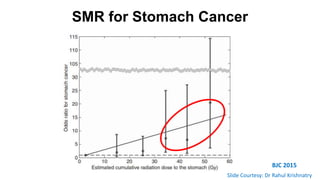
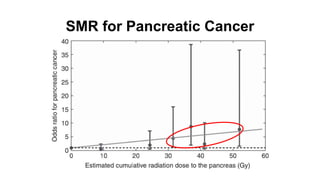
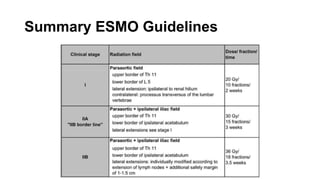
- For stage I seminoma, prophylactic radiation to the para-aortic lymph nodes is the standard of care to reduce the risk of recurrence.
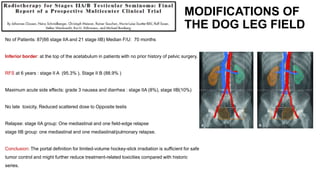
- For stage IIA/IIB seminoma, radiotherapy to the para-aortic, iliac, and inguinal lymph nodes is recommended, with 30Gy to the whole field and a 10Gy boost for stage IIA and 36Gy total for stage IIB.
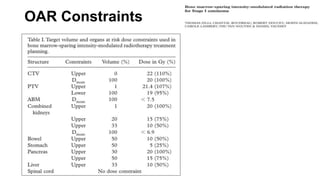
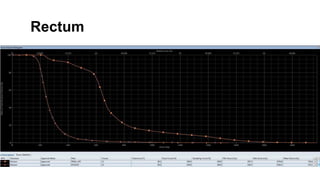
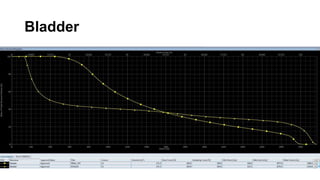
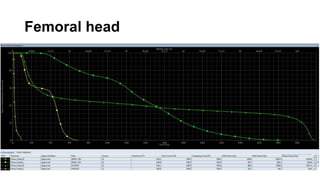
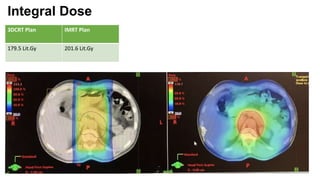
- Intensity modulated radiation therapy (IMRT) allows for improved sparing of