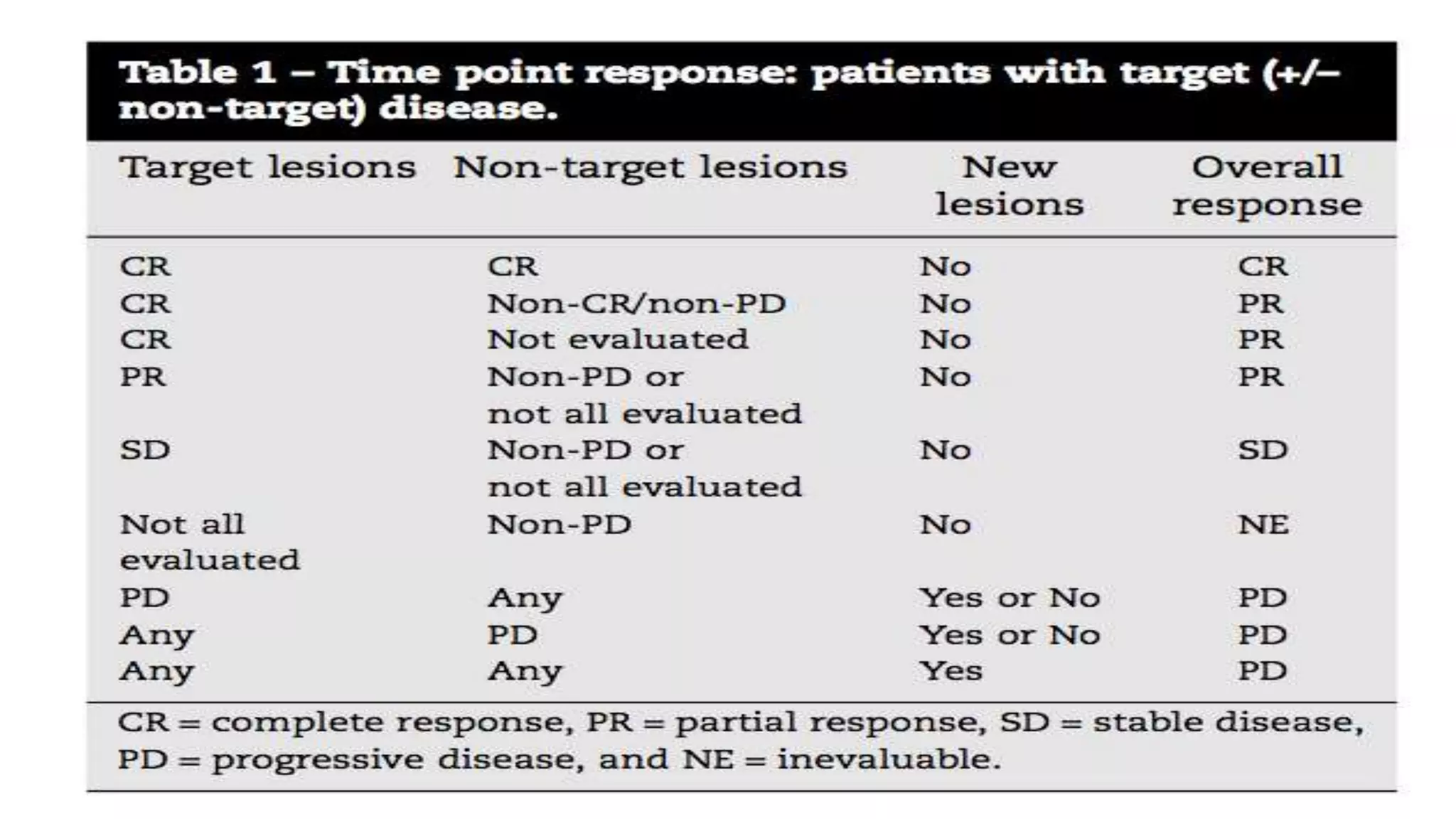
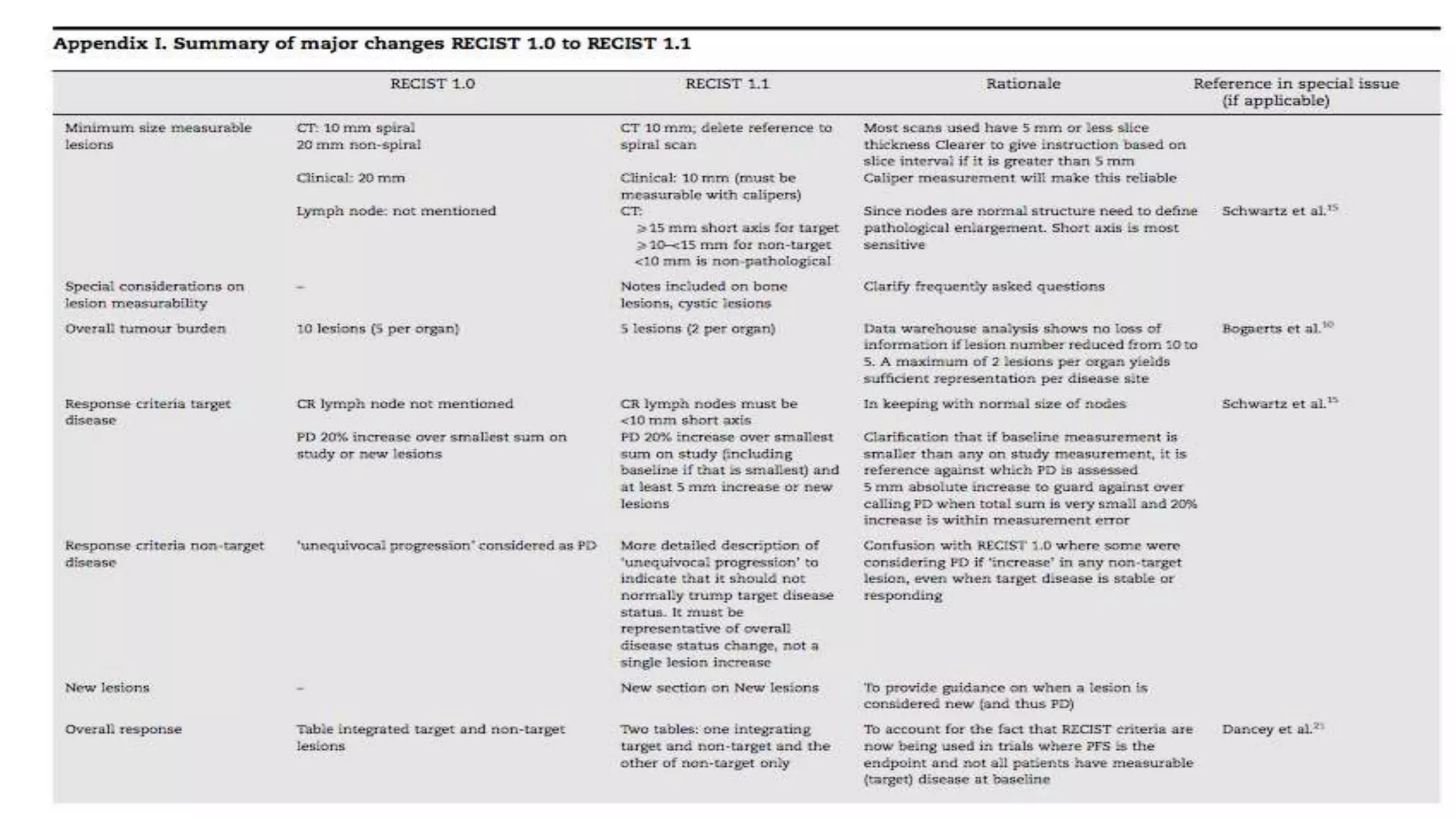
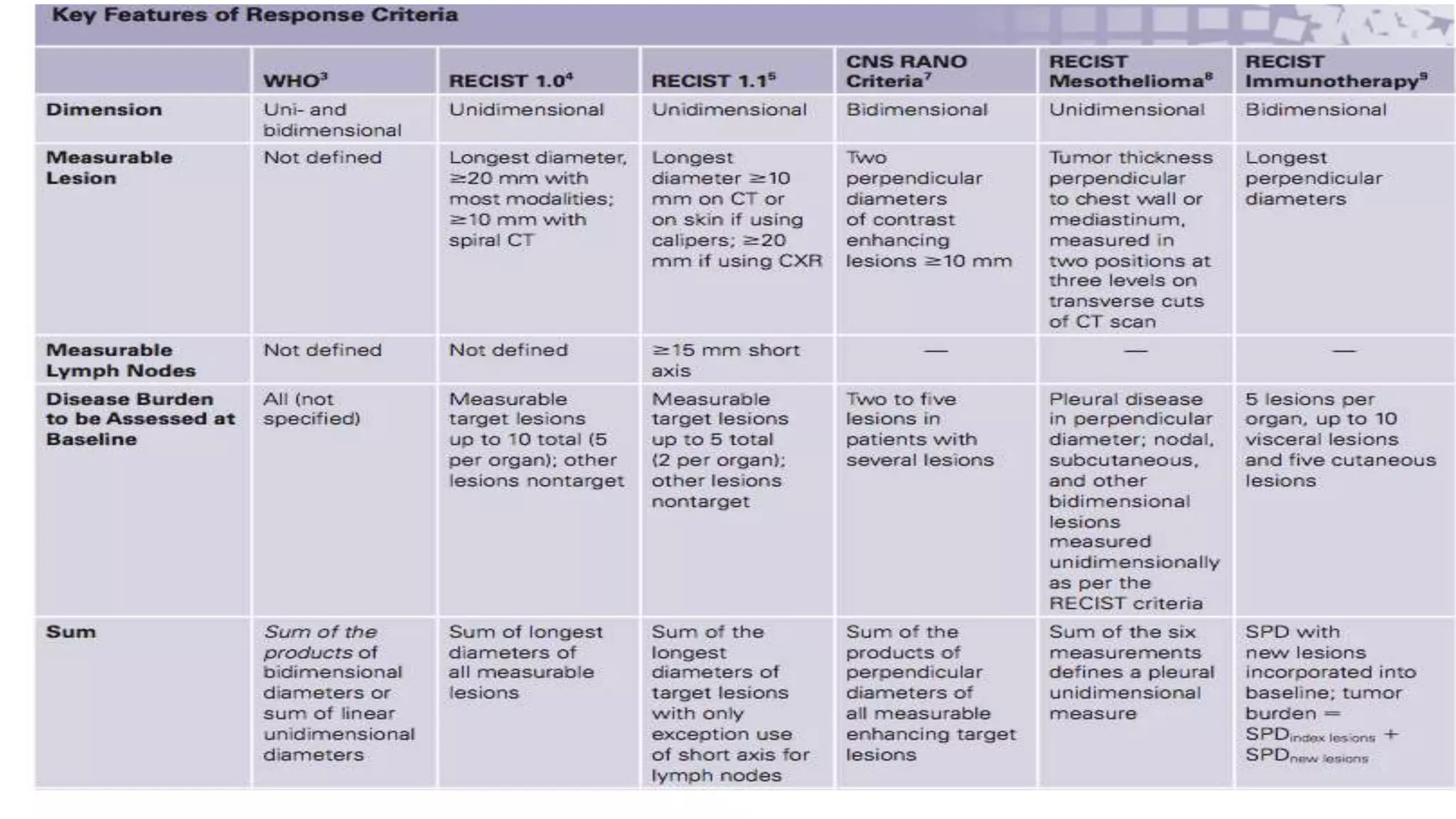
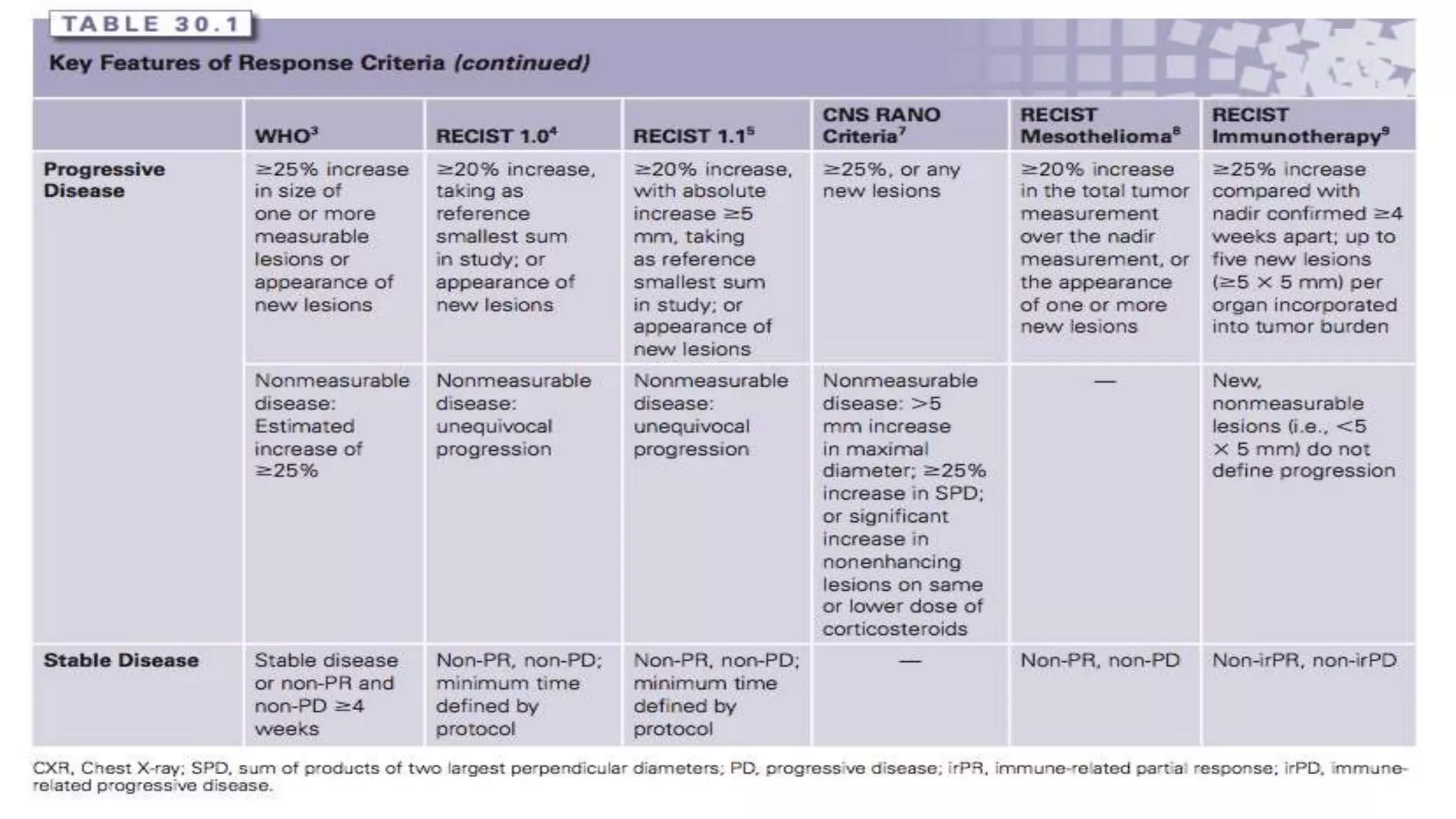
The RECIST guidelines provide standardized criteria for evaluating tumor response in cancer clinical trials. They were developed in 2000 by an international working group to simplify and standardize previous WHO response criteria. Key aspects of the RECIST guidelines include defining measurable and non-measurable lesions, criteria for complete response, partial response, stable disease and progressive disease based on tumor size measurements, and recommendations for frequency of tumor re-evaluation and confirming responses. The guidelines aim to facilitate objective and reproducible assessments of tumor burden and treatment response.