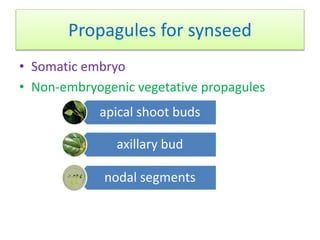
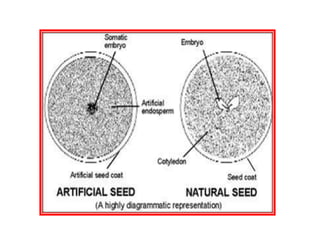
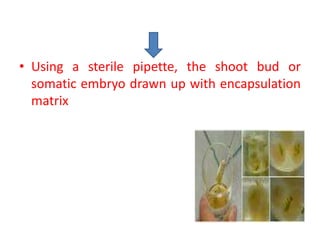
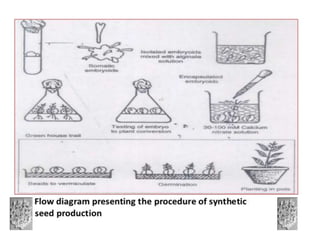
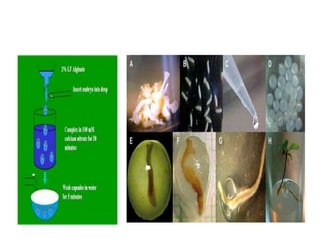
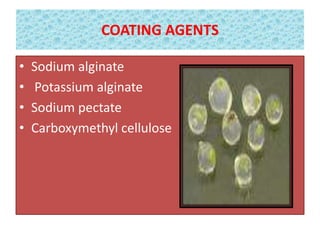
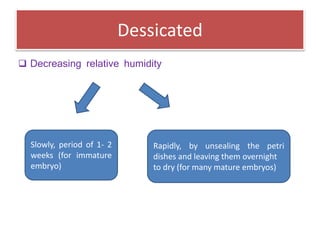
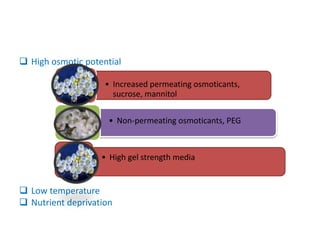
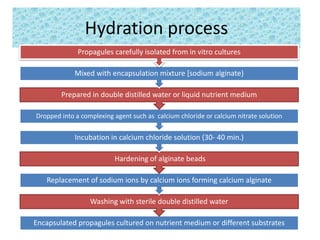
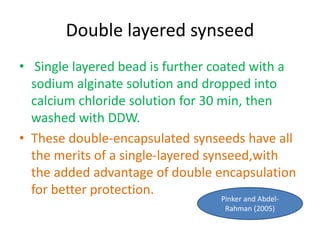
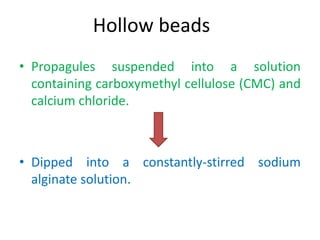
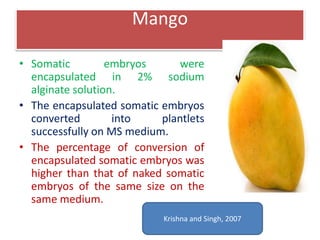
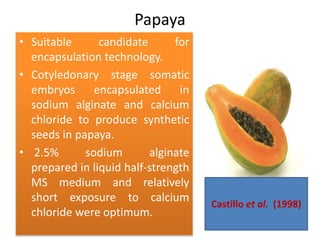
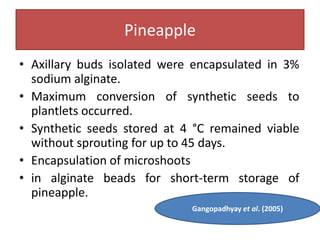
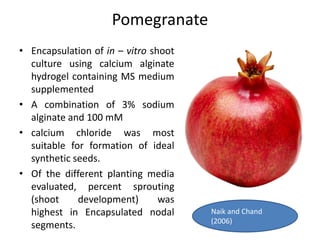
1) Synseed technology involves encapsulating plant propagules like somatic embryos, shoot tips, or buds in a gel matrix, usually sodium alginate, to create artificial seeds that can be stored and germinated like real seeds.
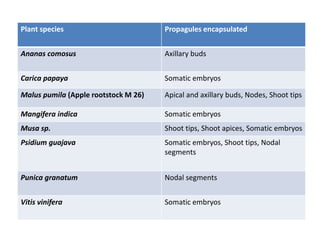
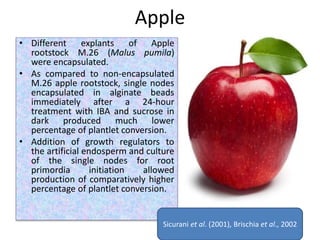
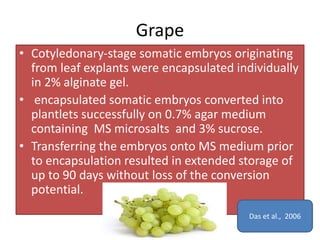
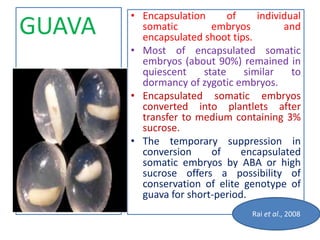
2) Various fruit crops have been successfully propagated using synseed technology including mango, banana, apple, grape, guava, and papaya. Somatic embryos and shoot tips are common propagules used.
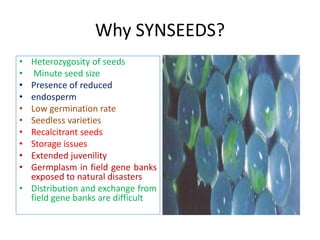
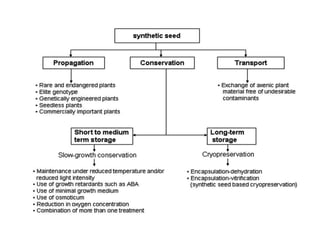
3) Advantages of synseed technology include maintaining genetic uniformity, facilitating long distance transportation and exchange of plant materials, and providing a means of propagation for hybrids, endangered species, and plants with recalcitrant seeds.