Nanotechnology can be used to clean the air through various applications. Some key points:
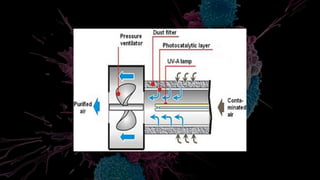
1. Nanoparticles like titanium dioxide can be used in photocatalytic filters to break down air pollutants like VOCs, NOx, and pathogens into harmless byproducts like water and carbon dioxide when exposed to light.
2. Other nanomaterials like gold-embedded manganese oxide have been shown to effectively remove common indoor air pollutants like acetaldehyde, toluene, and hexane at room temperature.
3. Nanotechnology allows more effective air filtration and purification systems to be developed for indoor and outdoor applications to improve air quality.