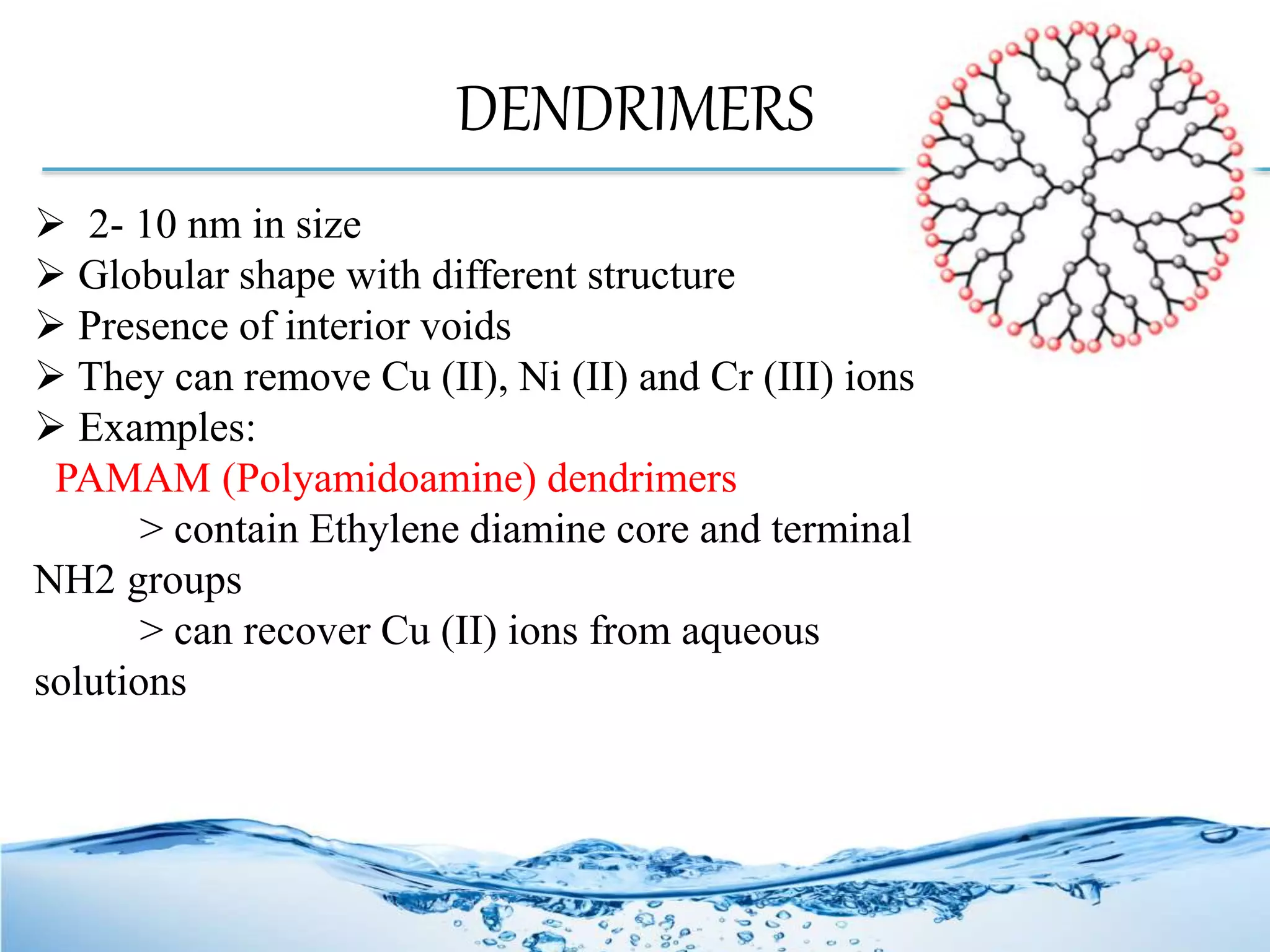
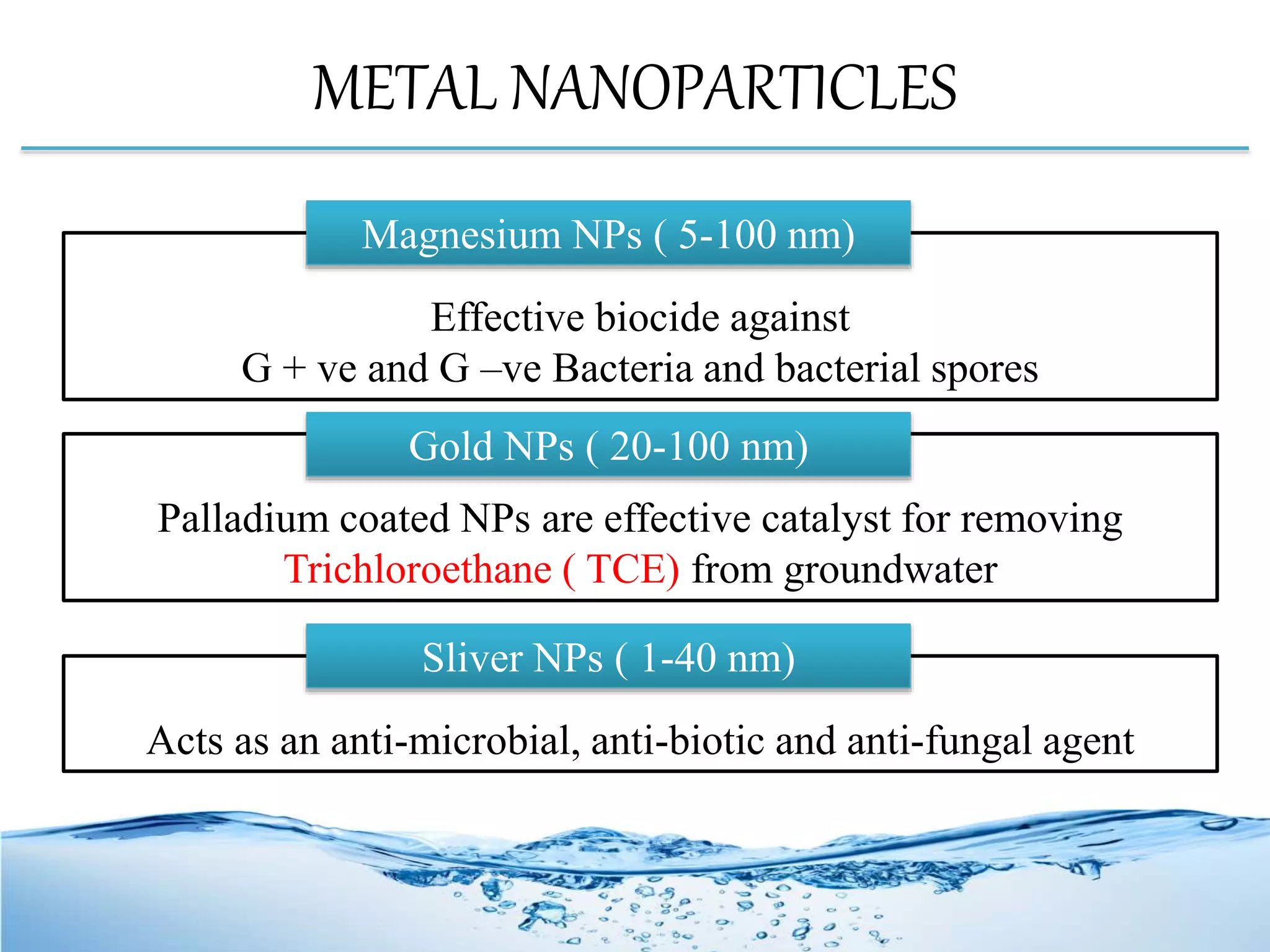
Nanotechnology plays a crucial role in water treatment through the use of nanomaterials that enhance the performance of purification processes by efficiently removing pollutants like toxic metals and microbes. Various types of nanomaterials, including nanoparticles, dendrimers, and zeolites, offer advanced methods such as nanosorption, nanofiltration, and photocatalysis to treat both wastewater and drinking water. These methods benefit from unique properties like high surface area and reactivity, leading to more precise and effective water treatment solutions.