Nanotechnology shows promise for protecting the environment in several ways:
1. Nanoparticles can be used to make more efficient solar cells, wind turbine blades, and batteries to help transition to renewable energy.
2. Nanomaterials have properties that allow for more effective water treatment, such as through filtration and photocalytic disinfection.
3. Nanocatalysts can help reduce pollution by enabling more effective catalytic converters and chemical production processes.
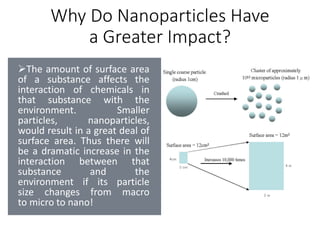
However, there is still uncertainty around the environmental impacts of nanomaterials. Their large surface area means nanoparticles could interact with the environment in unexpected ways, and some core nanomaterials are toxic. More research is needed to evaluate risks and ensure nanotechnologies are developed and managed