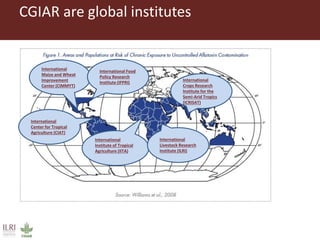
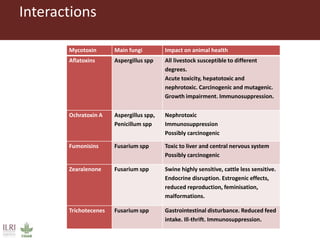
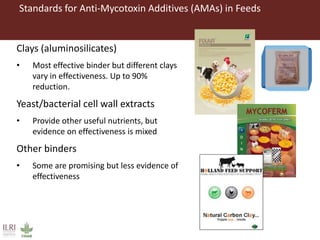
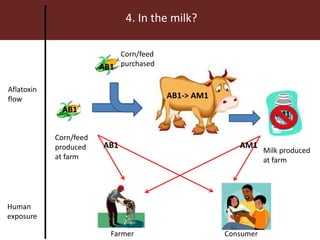
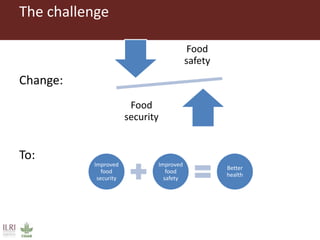
The document discusses the impact of mycotoxins, particularly aflatoxins, on livestock in low and middle-income countries, focusing on Kenya's dairy industry. It highlights the health risks posed to animals and humans, the socioeconomic factors influencing exposure, and the need for effective mitigation strategies throughout the dairy value chain. Various approaches for reducing aflatoxin contamination are outlined, emphasizing the importance of regulatory standards and consumer awareness.