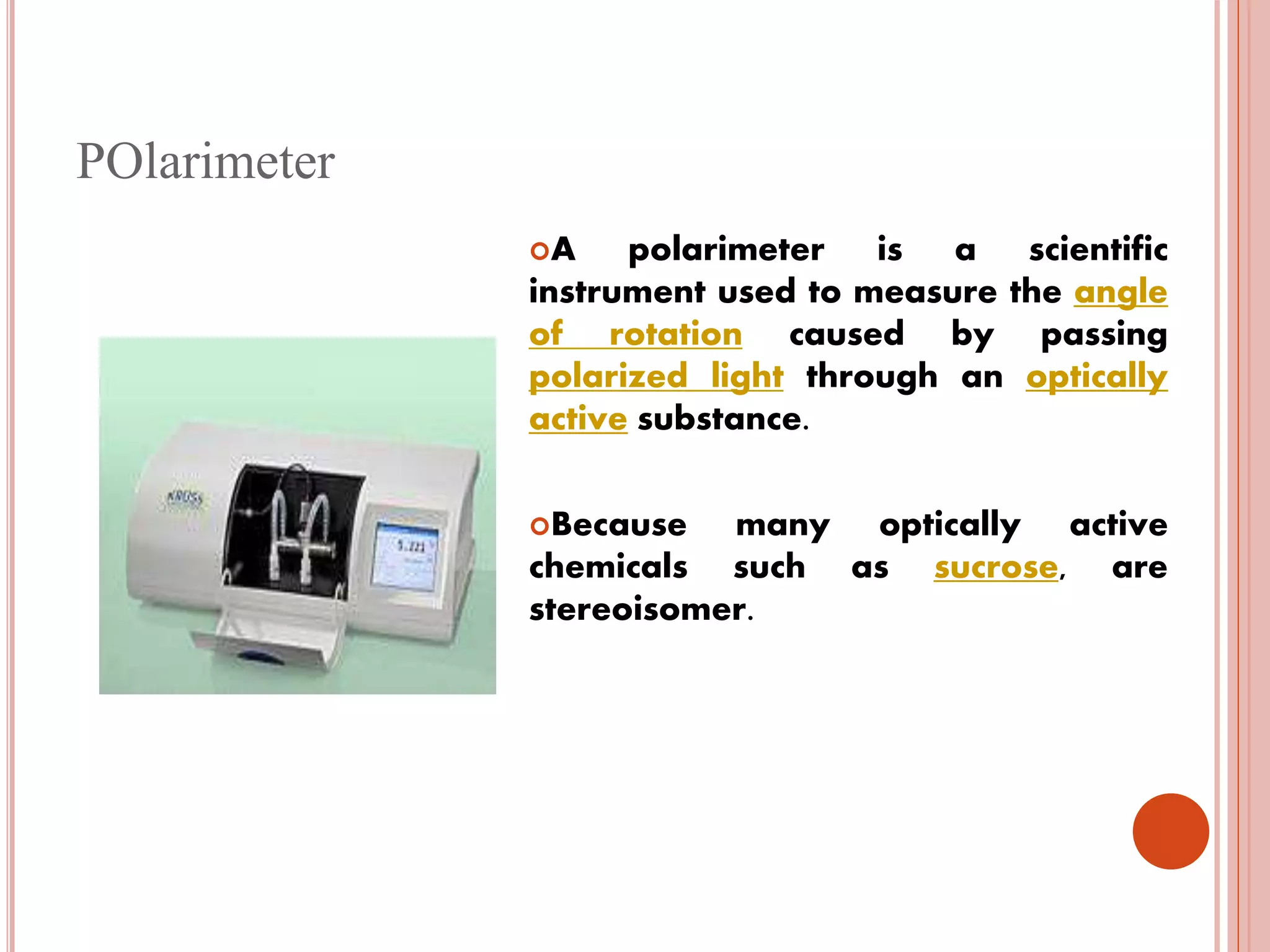
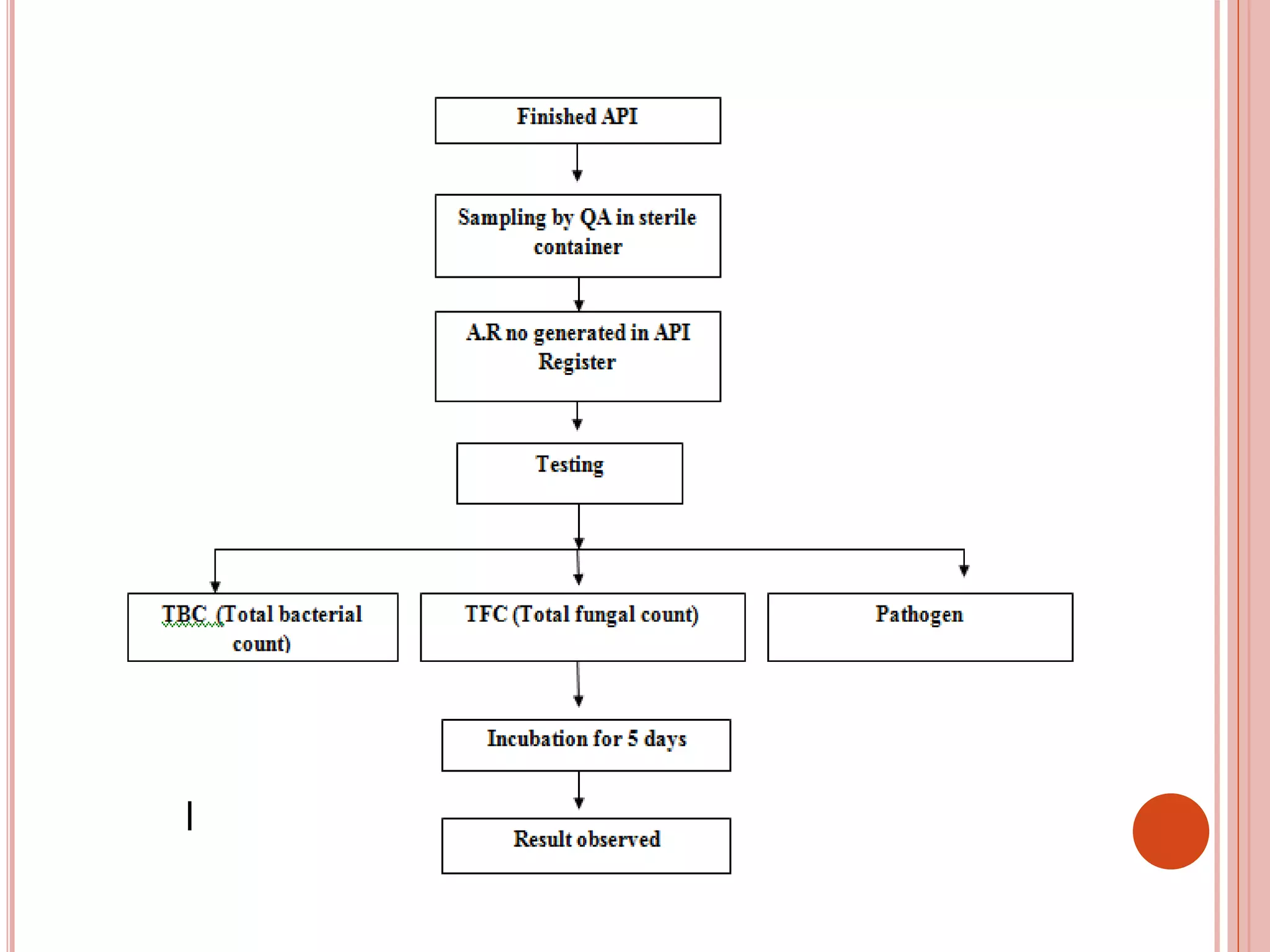
The document describes an industrial training at Zydus Cadila Healthcare Ltd in Ankleshwar, India. It discusses the company's vision, the active pharmaceutical ingredients (APIs) manufactured, and an overview of the quality control department and its various sections. The quality control department ensures raw materials and finished products meet specifications through testing and analysis using instruments like HPLC, GC, and microbiological analysis. The training provided insight into good manufacturing practices and quality control processes required in the pharmaceutical industry.