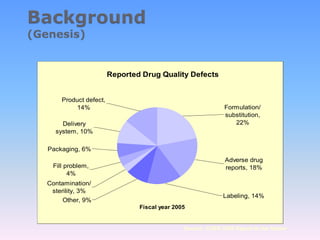
The document discusses FDA's quality system approach to cGMP regulations. It outlines six quality systems that FDA expects companies to have in place: quality, facilities and equipment, materials, production, laboratory, and packaging and labeling. The goal is to encourage a proactive, risk-based approach focused on critical processes to ensure product quality and safety.