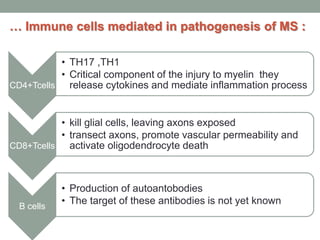
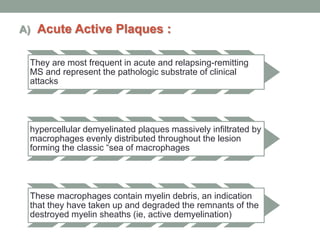
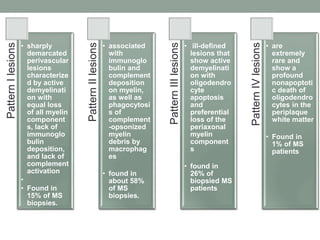
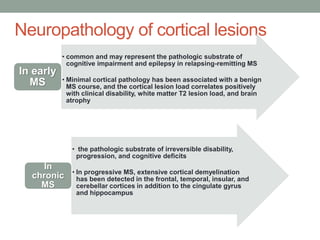
Multiple sclerosis (MS) is a chronic inflammatory disease that affects the central nervous system. It has four clinical forms and is more common in females than males. The exact cause is unknown but is believed to involve both genetic and environmental factors. Pathologically, MS is characterized by lesions throughout the white matter of the brain and spinal cord caused by inflammation and the loss of myelin. These lesions can be either actively demyelinating or chronically demyelinated. While the disease course and prognosis can vary between individuals, it generally involves periods of relapse followed by remission or a progressive decline over time.