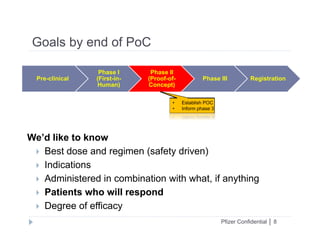
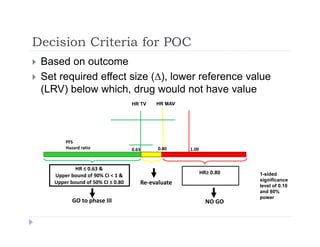
This document discusses clinical proof-of-concept (POC) trials in drug development. It defines POC as establishing whether a drug is reasonably likely to succeed based on early evidence of safety and efficacy. The document outlines goals of POC trials, decision criteria used, and strategies to improve probability of success such as better patient selection using biomarkers. It provides examples of oncology POC trials and discusses practical considerations for using patient selection approaches.