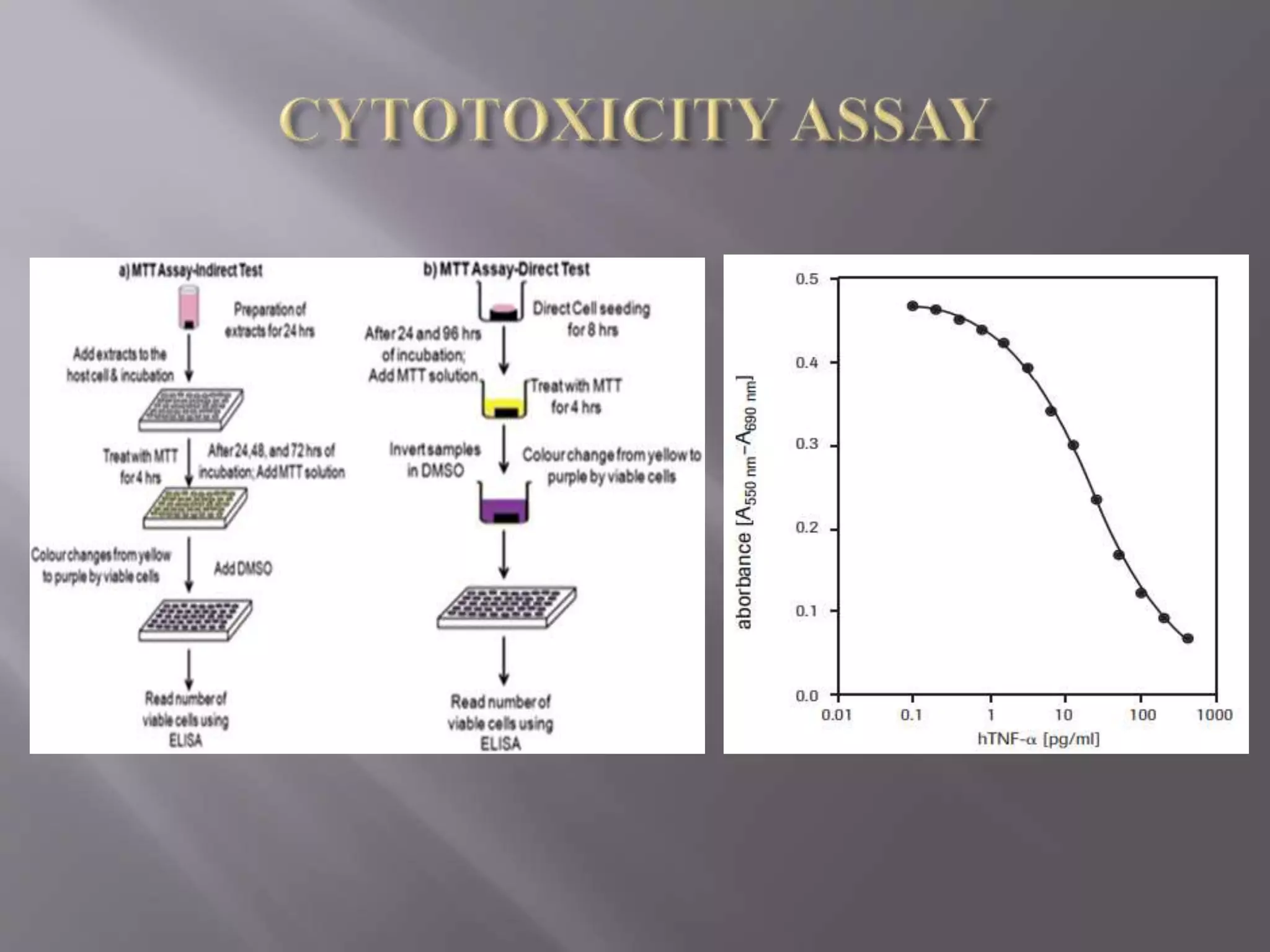
The document discusses the principles and applications of MTT cell viability assays. The MTT assay is a colorimetric assay that measures the reduction of MTT by mitochondrial succinate dehydrogenase in metabolically active cells to form an insoluble purple formazan product. The amount of formazan produced is directly proportional to the number of viable cells and can be used to measure cell proliferation, viability, and cytotoxicity in response to drugs or other factors. The MTT assay is inexpensive, rapid, quantitative, and reproducible, making it well-suited for applications like cytotoxicity testing, drug screening, and measuring responses to growth factors.


![ The cells are then solubilized with an organic solvent (e.g.:
Isopropanol /dimethyl sulfoxide[DMSO]) and the released,
solubilized formazan reagent is measured
spectrophotometrically.
Since reduction of MTT can only occur in metabolically
active cells the level of activity is a measure of the viability
of the cells.](https://image.slidesharecdn.com/mttassay-220120024341/75/PRINCIPLES-AND-APPLICATIONS-OF-CELL-VIABILITY-ASSAY-MTT-ASSAY-3-2048.jpg)