Embed presentation
Download to read offline
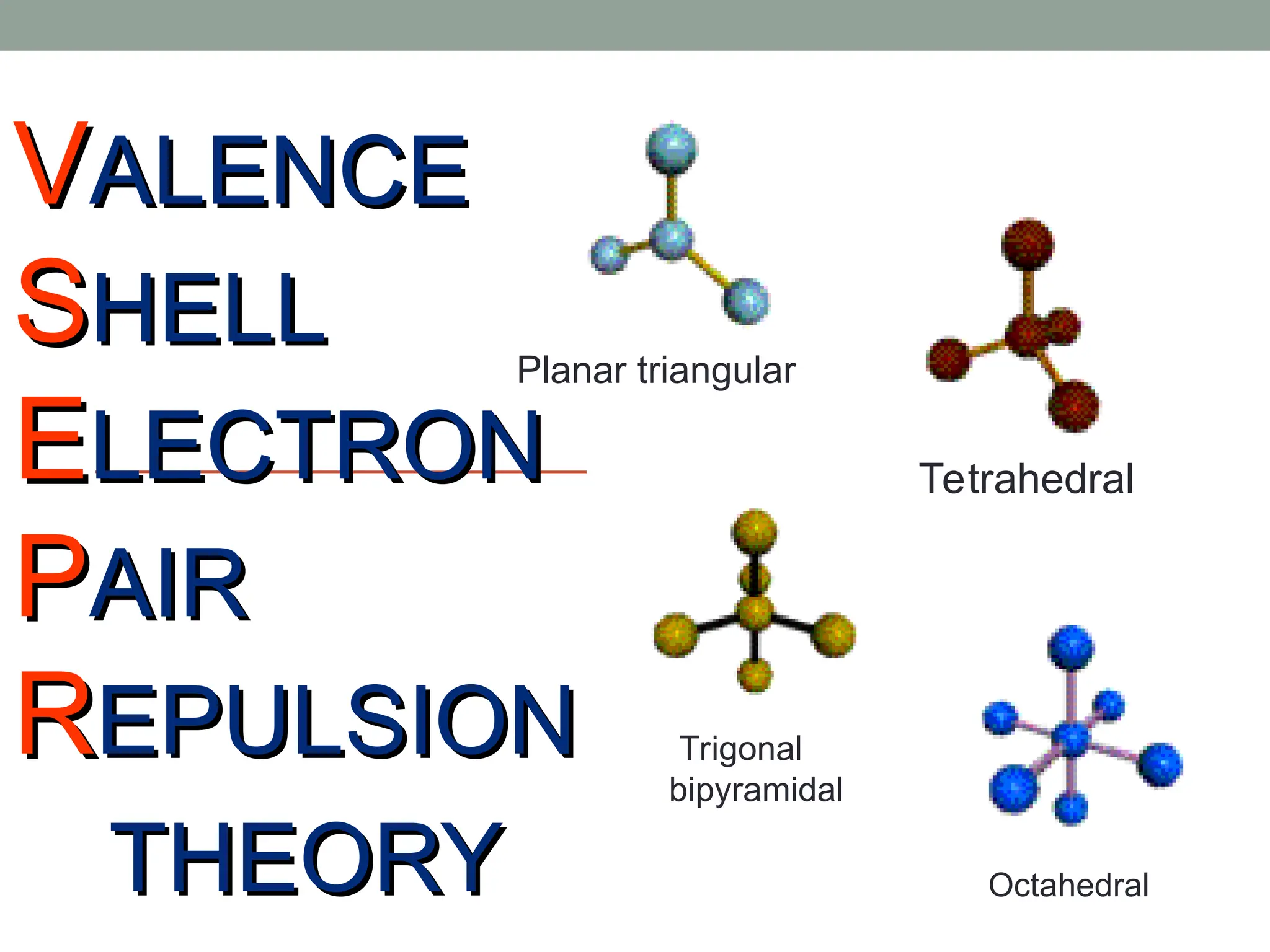


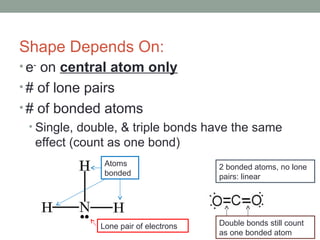
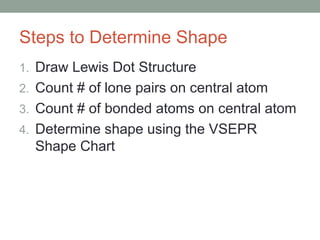
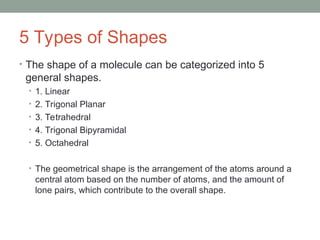
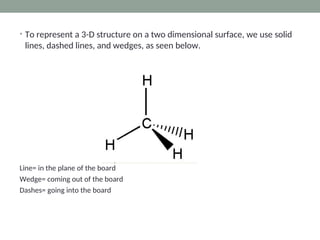
The document explains Valence Shell Electron Pair Repulsion (VSEPR) theory, which is used to predict the molecular geometry of covalent compounds and polyatomic ions based on the arrangement of electron pairs around a central atom. It outlines steps for determining a molecule's shape, including drawing Lewis dot structures and counting lone pairs and bonded atoms. The document categorizes molecular shapes into five types: linear, trigonal planar, tetrahedral, trigonal bipyramidal, and octahedral.






