The document discusses molecular distillation, which is used for purifying oils and separating vitamins, with a focus on the importance of the mean free path of molecules. It compares packed and plate columns for distillation processes, outlining advantages and disadvantages including efficiency, cost, and suitability for different flow rates. Additionally, it details the design procedure for packed columns and methods for determining column height based on theoretical and transfer unit methods.




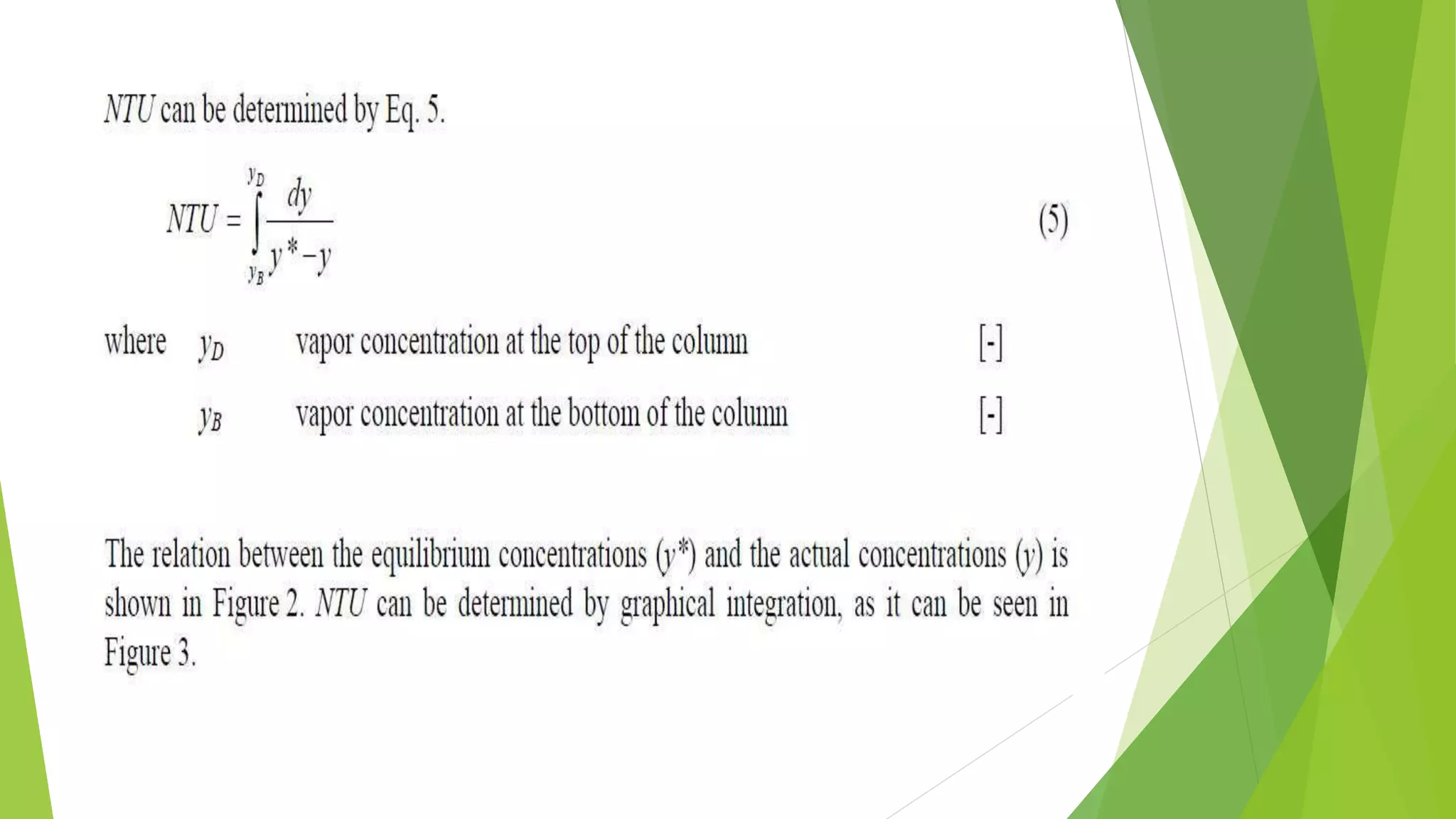
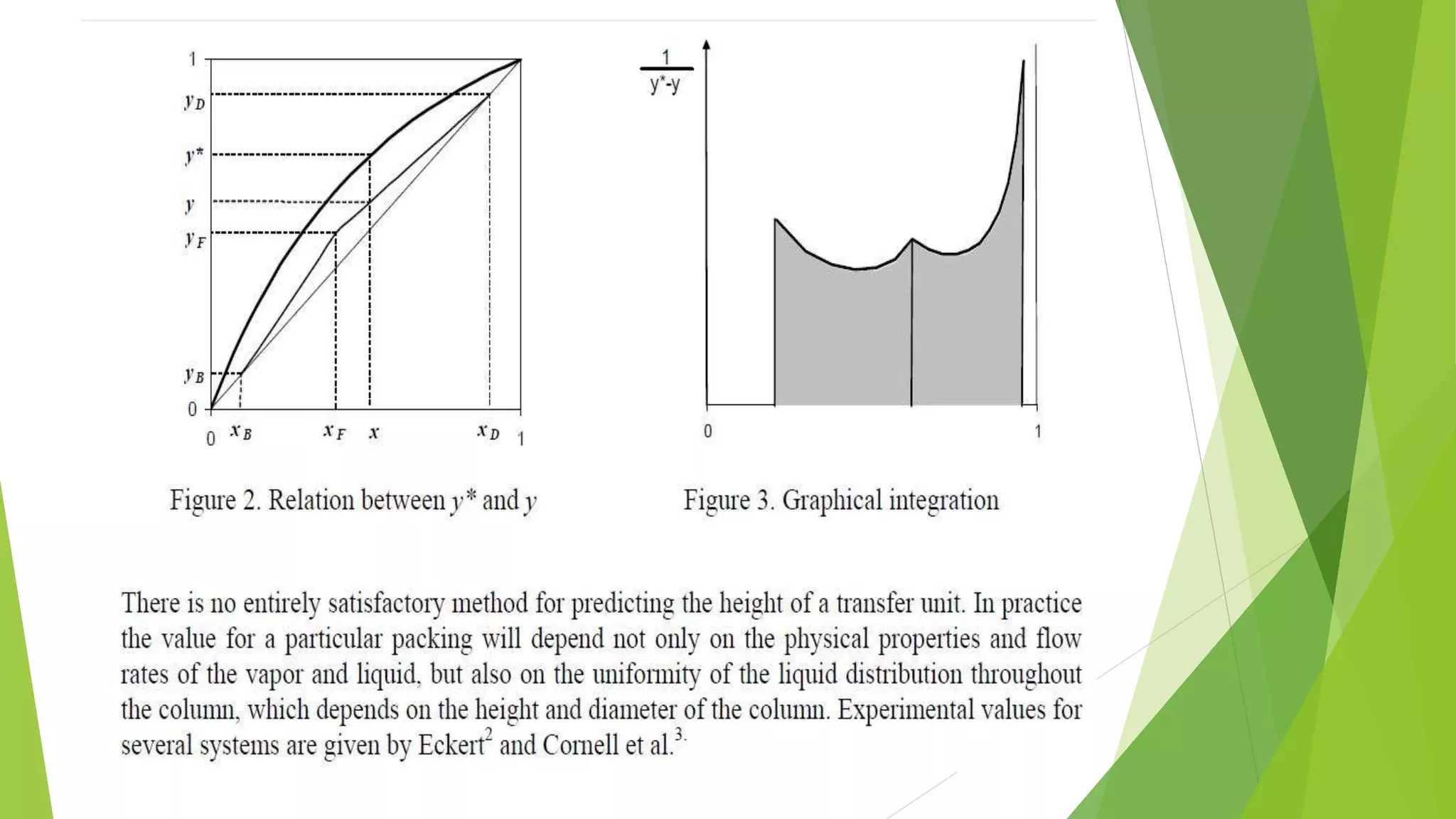
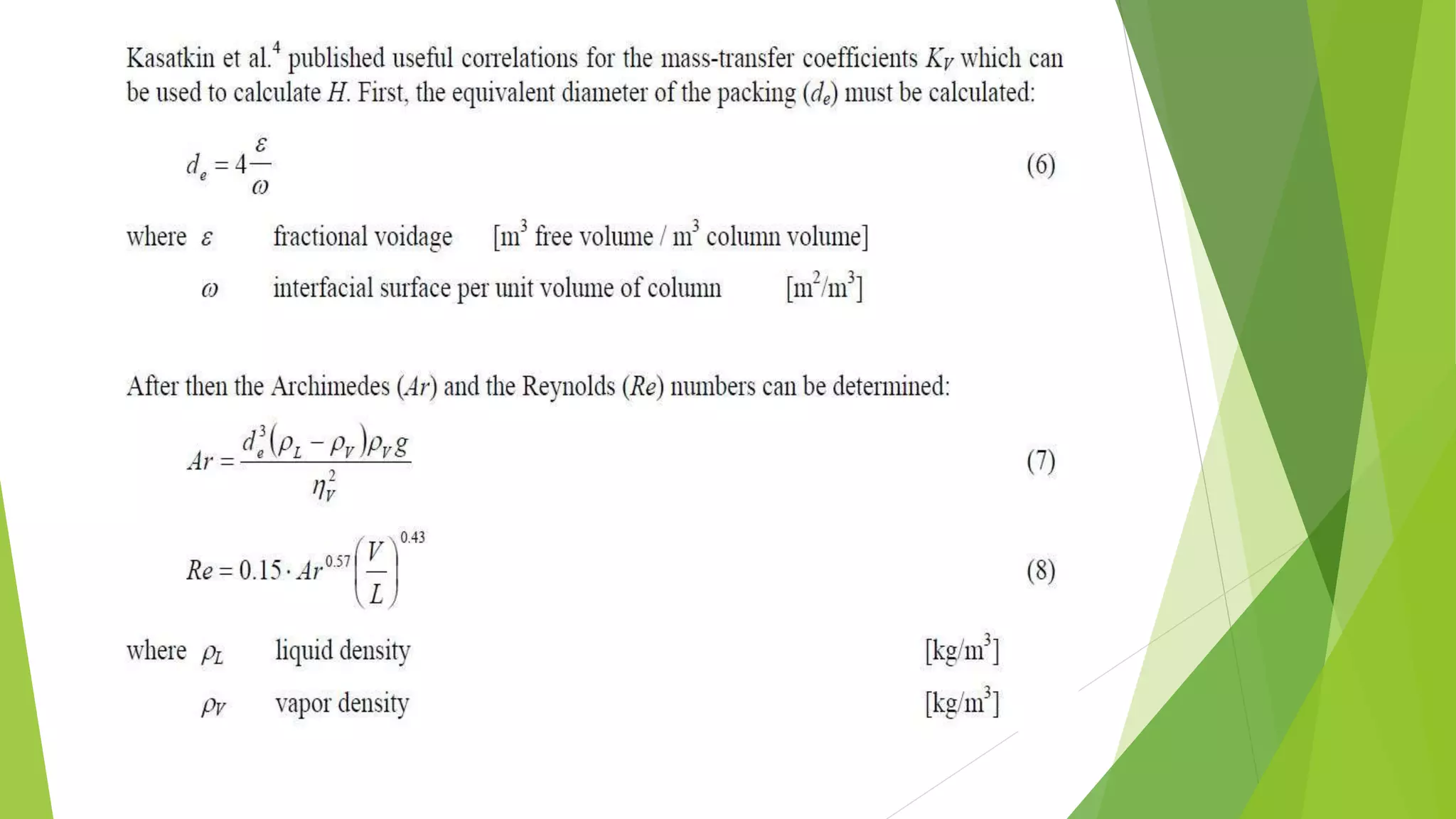
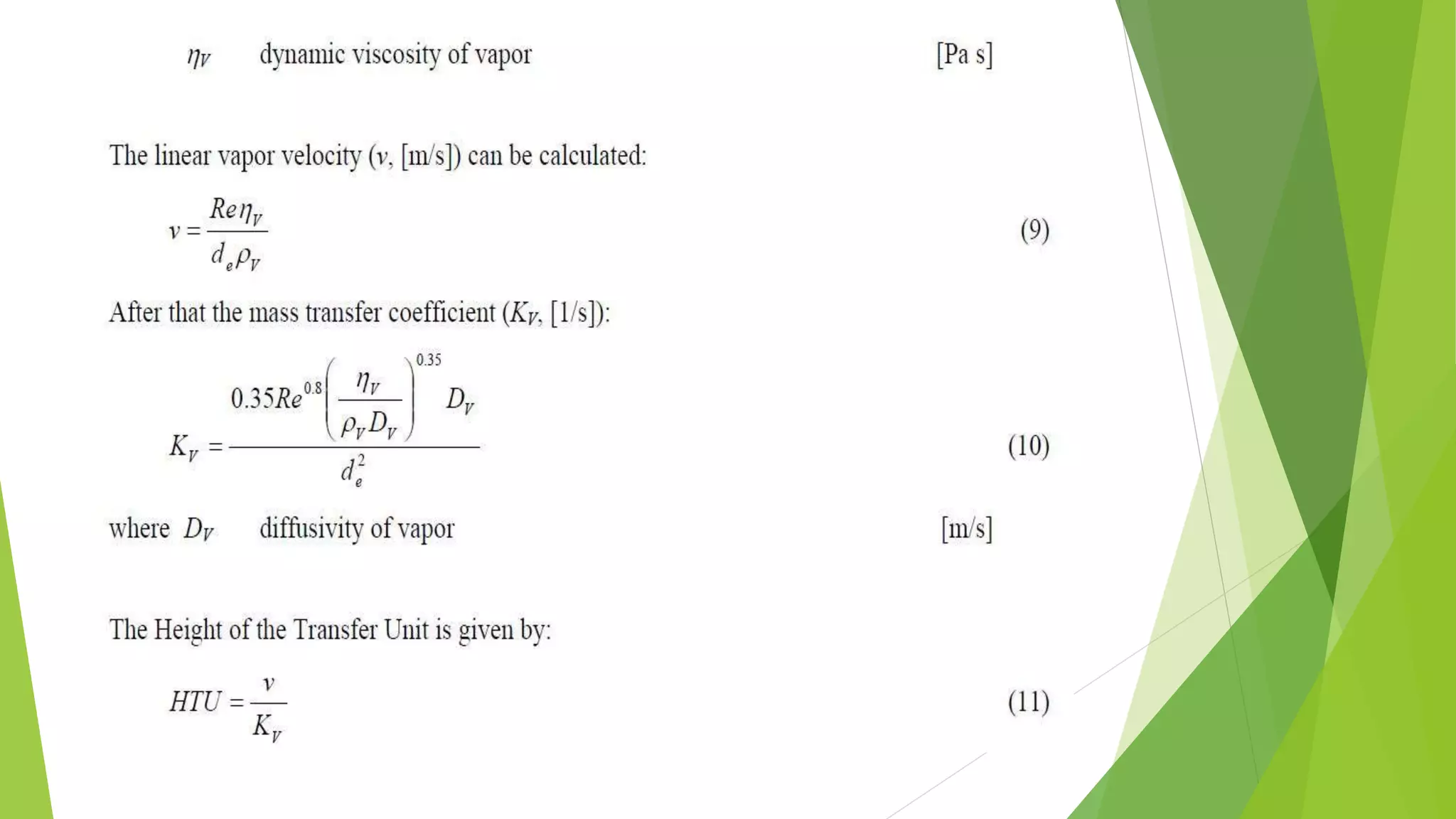
![1.2. The transfer unit method
The transfer unit is a part of column height
where the change in vapor concentration
equals tothe average driving force. The driving
force is the difference between the
equilibrium (y*) andthe actual (y) vapor
concentration.
The column height can be computed by Eq. 4.
H = HTU ⋅ NTU
_________________________(4)
where HTU Height of a Transfer Unit [m]
NTU Number of Transfer Units [-]](https://image.slidesharecdn.com/masstransfer-141117024829-conversion-gate02/75/molecular-distillation-and-packed-columm-distillation-10-2048.jpg)