Embed presentation
Download to read offline
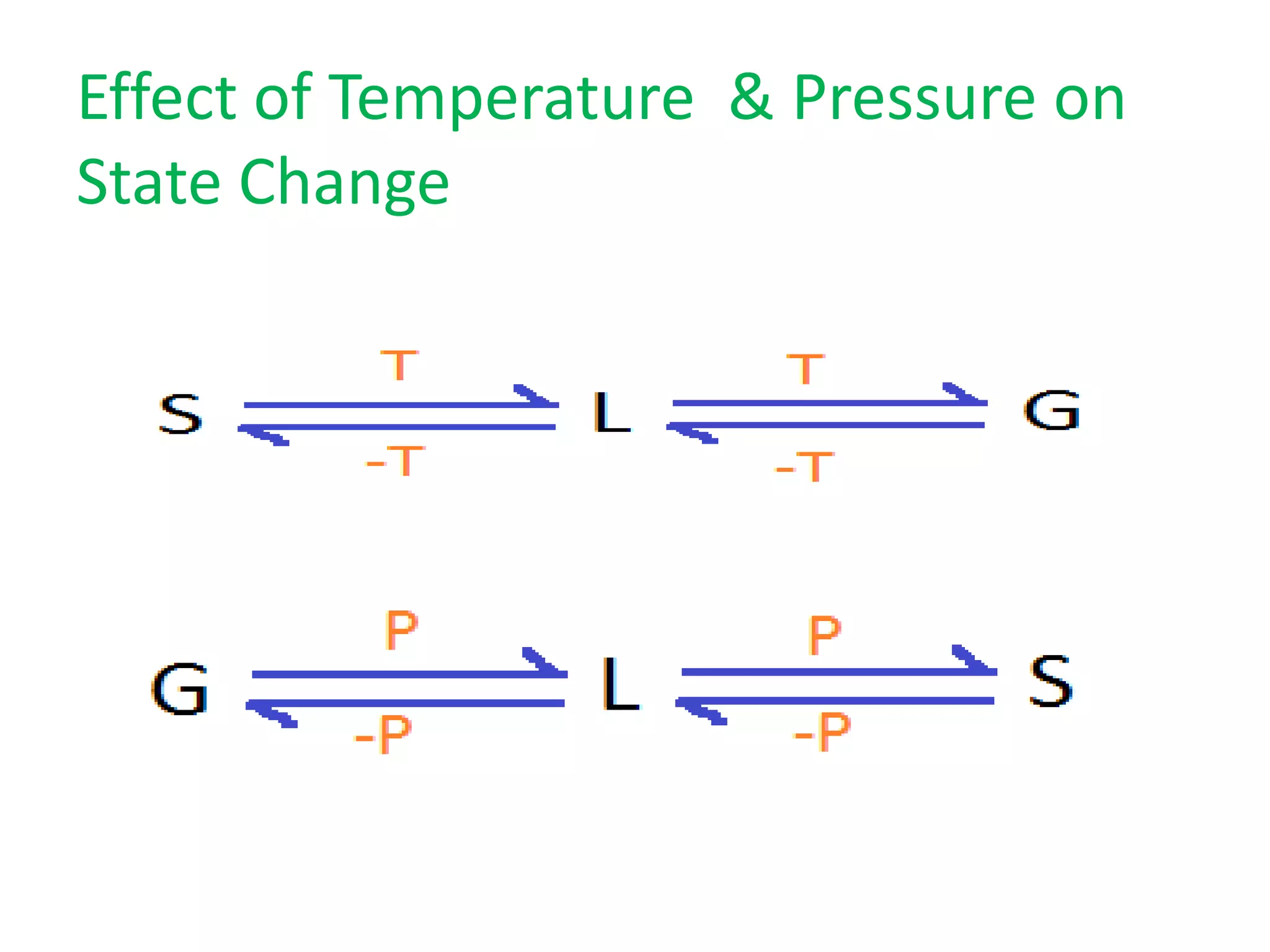
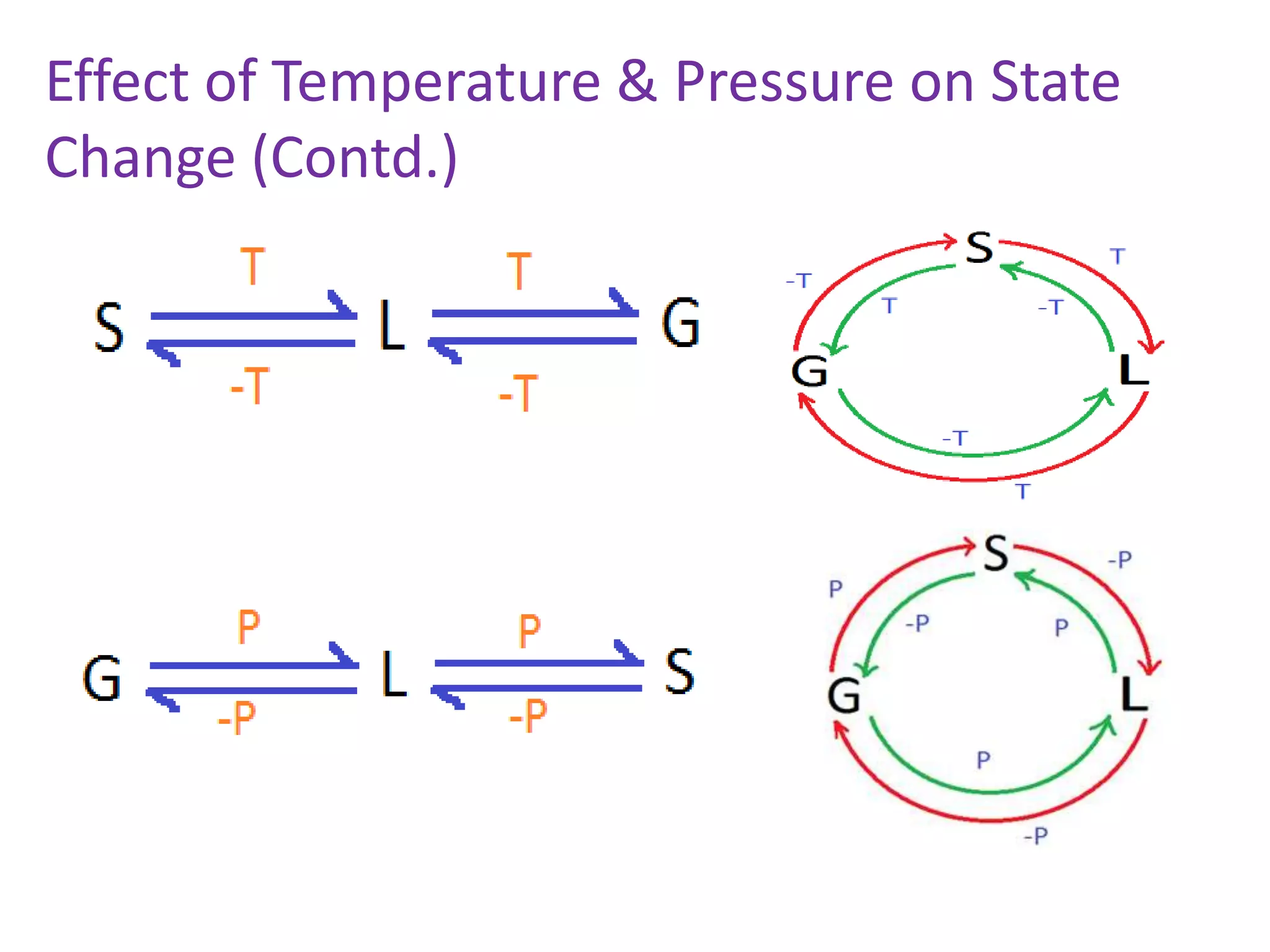
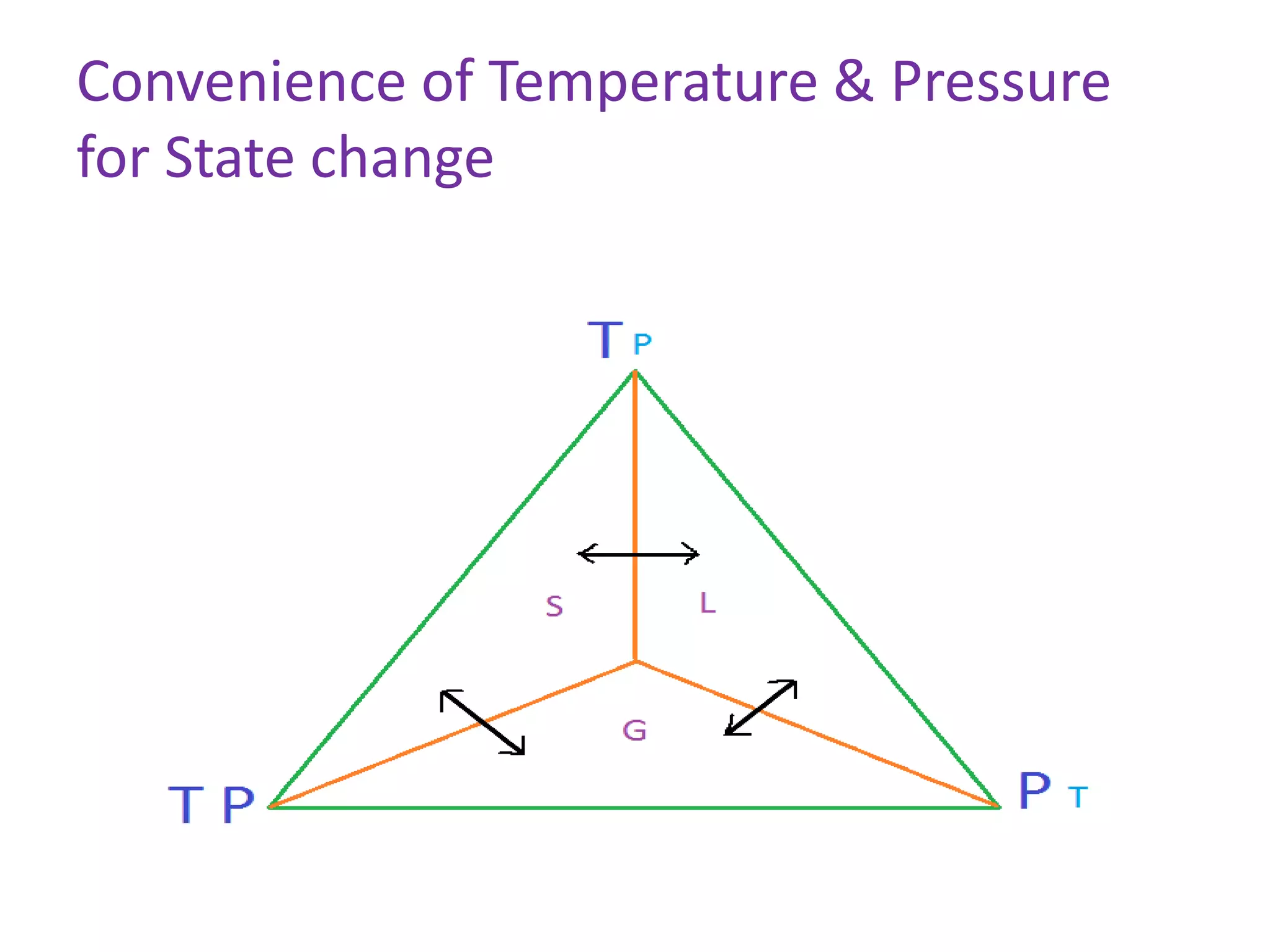
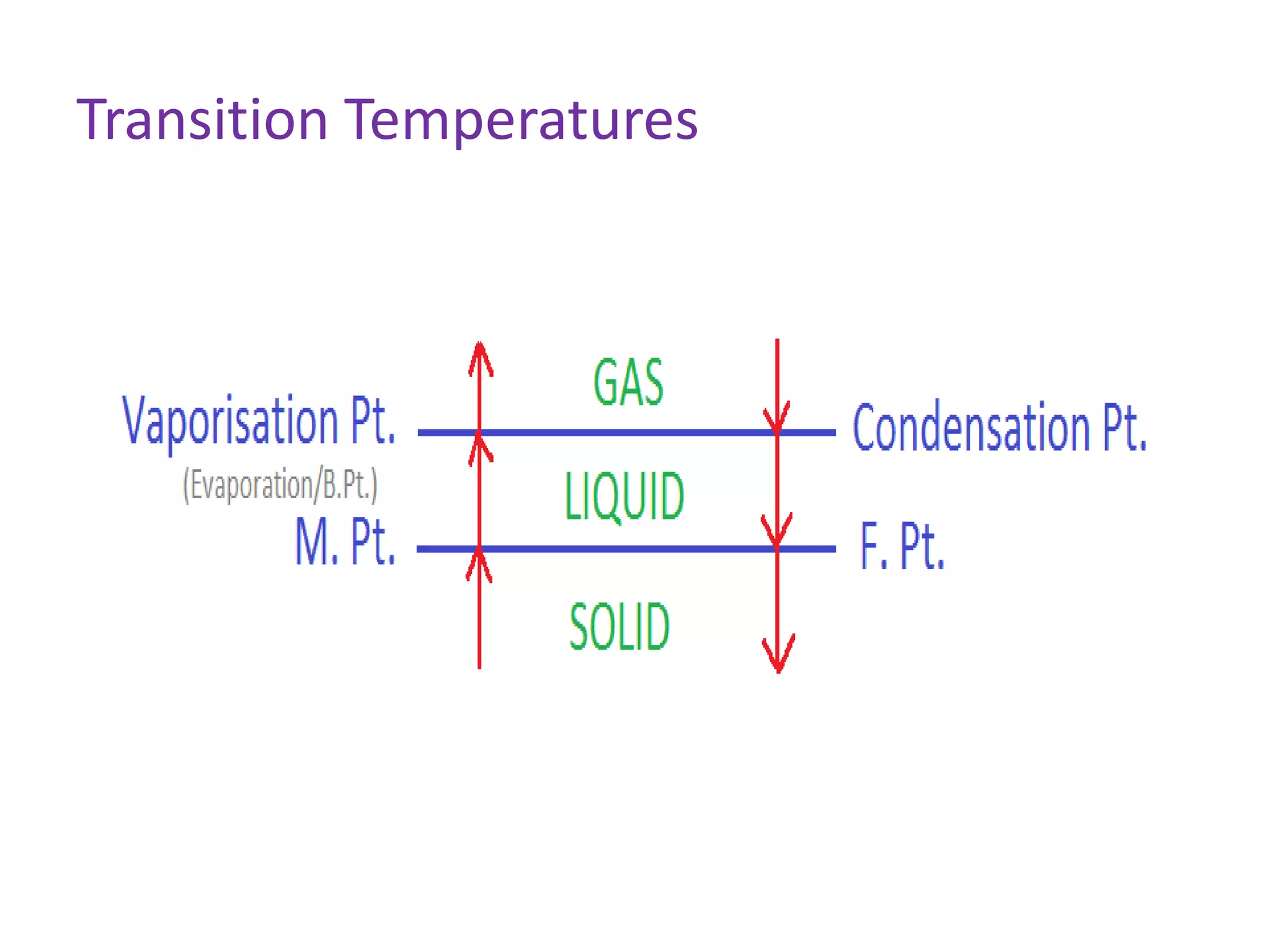
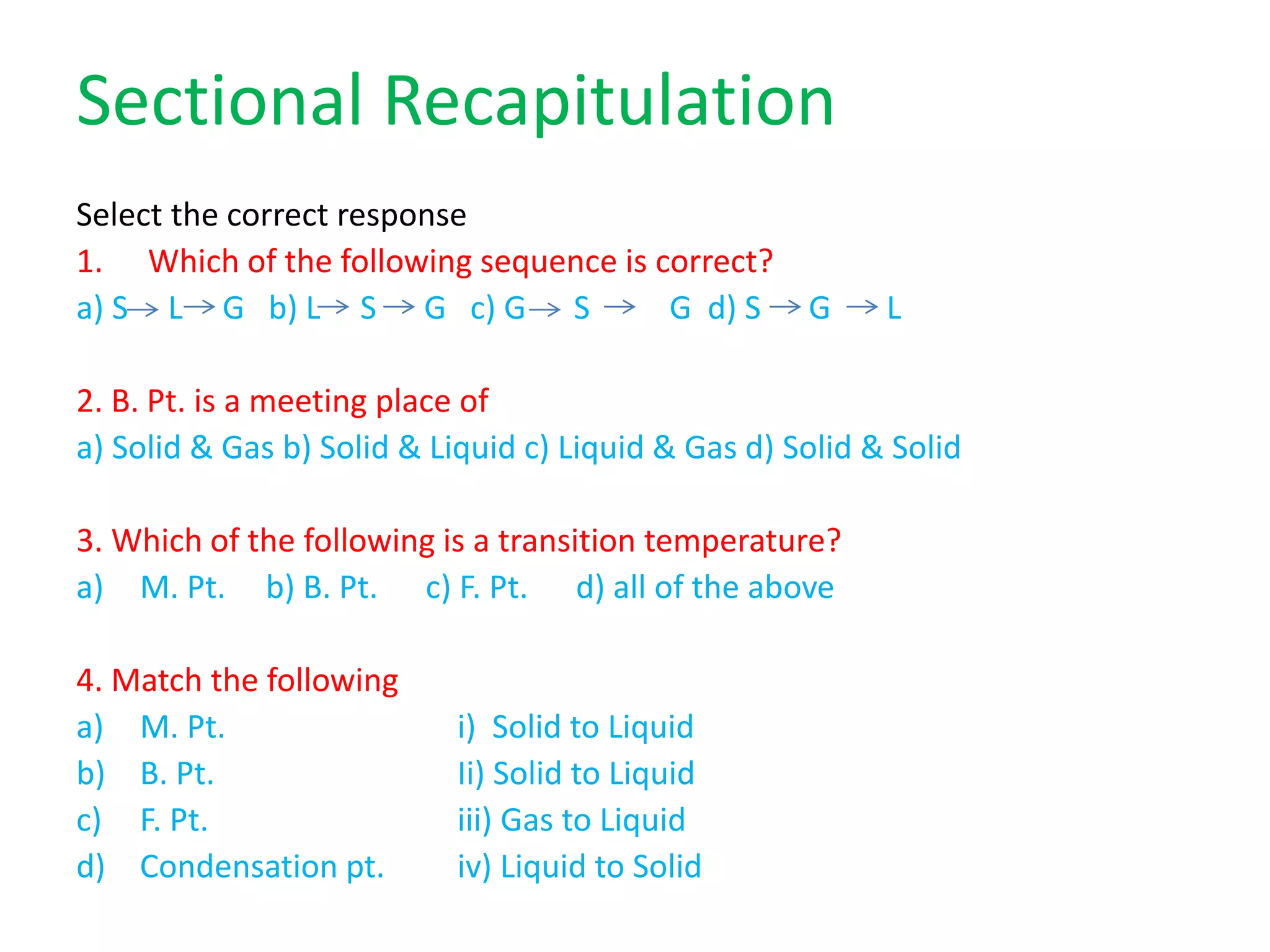
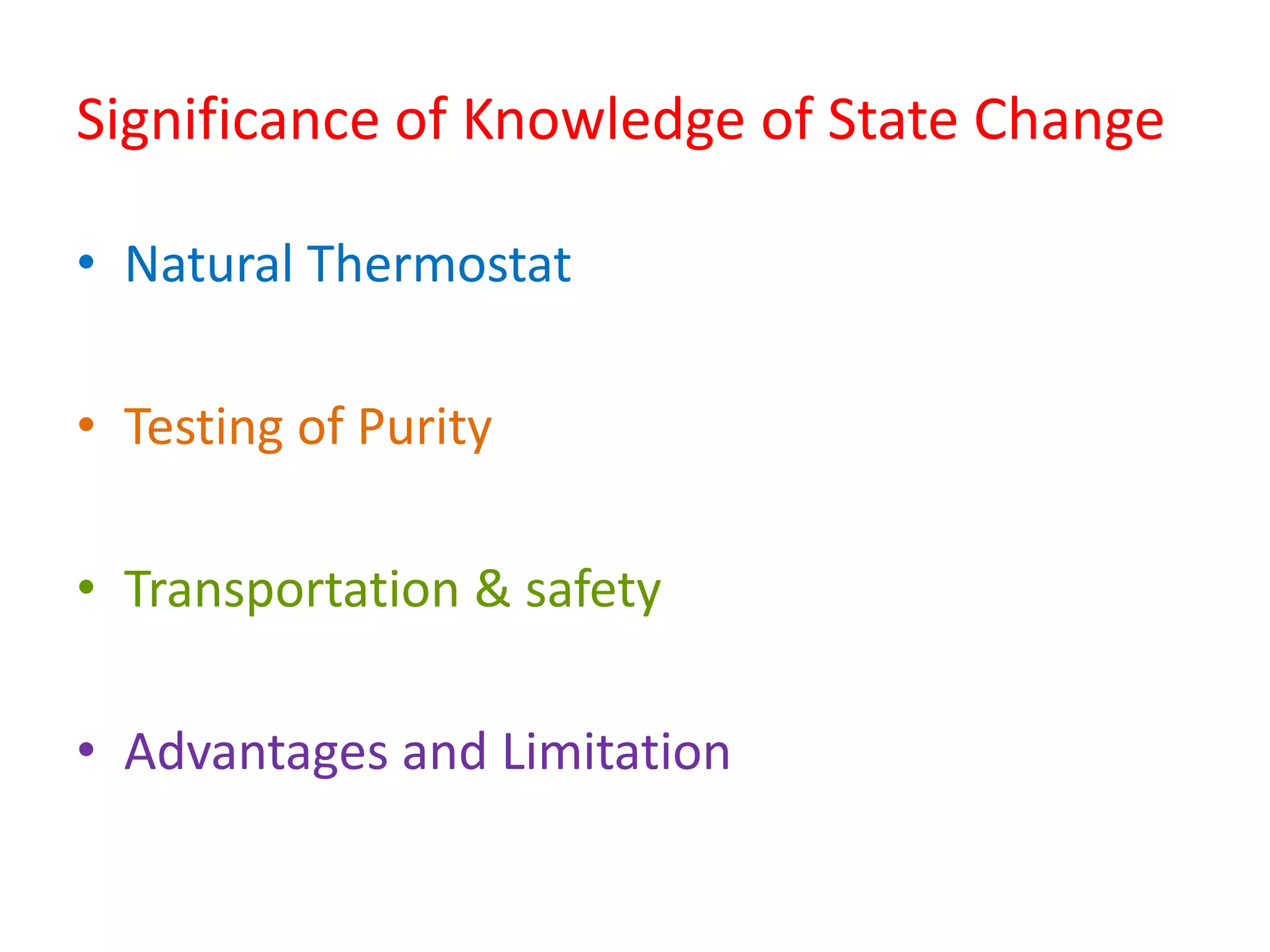
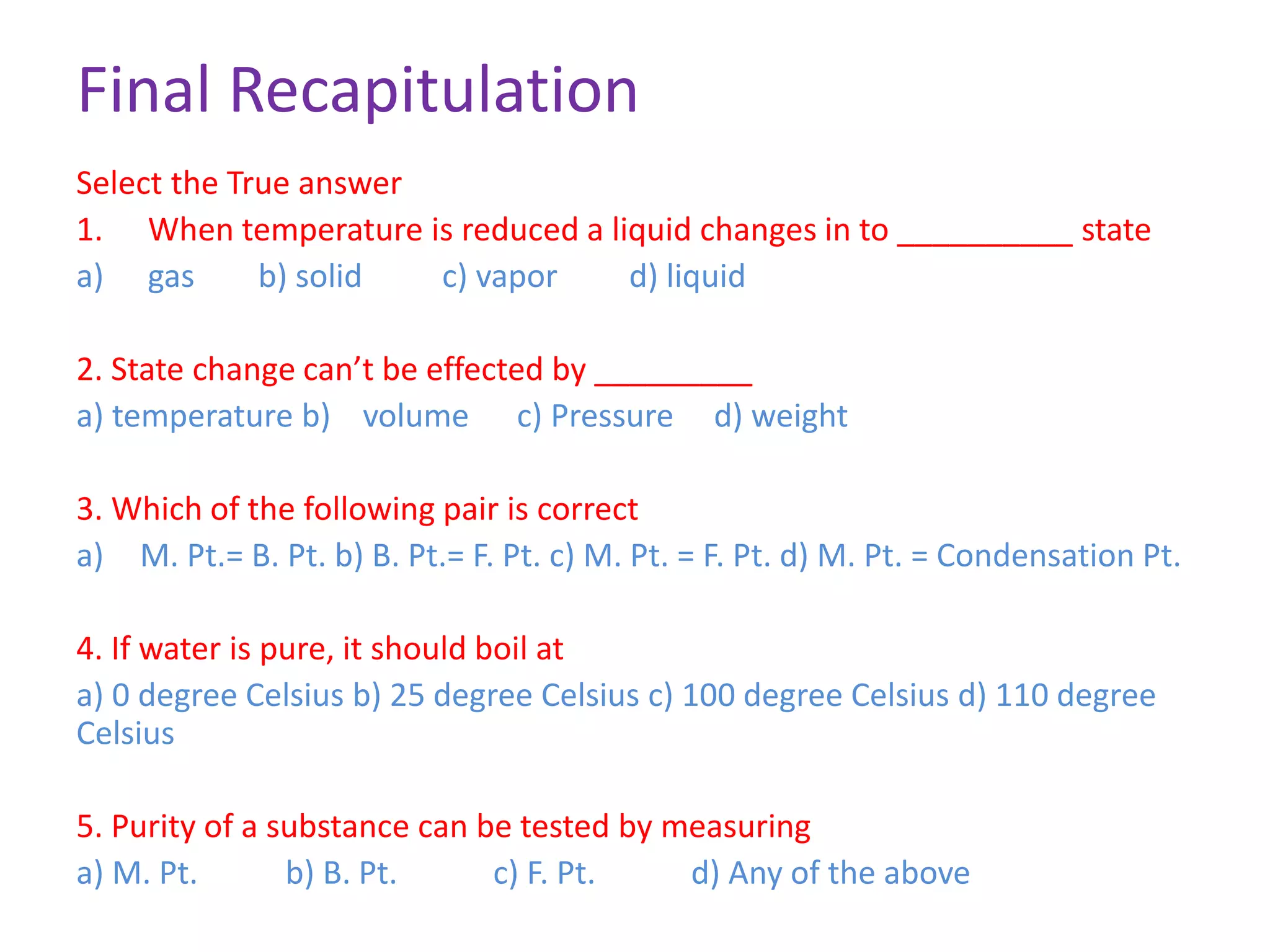
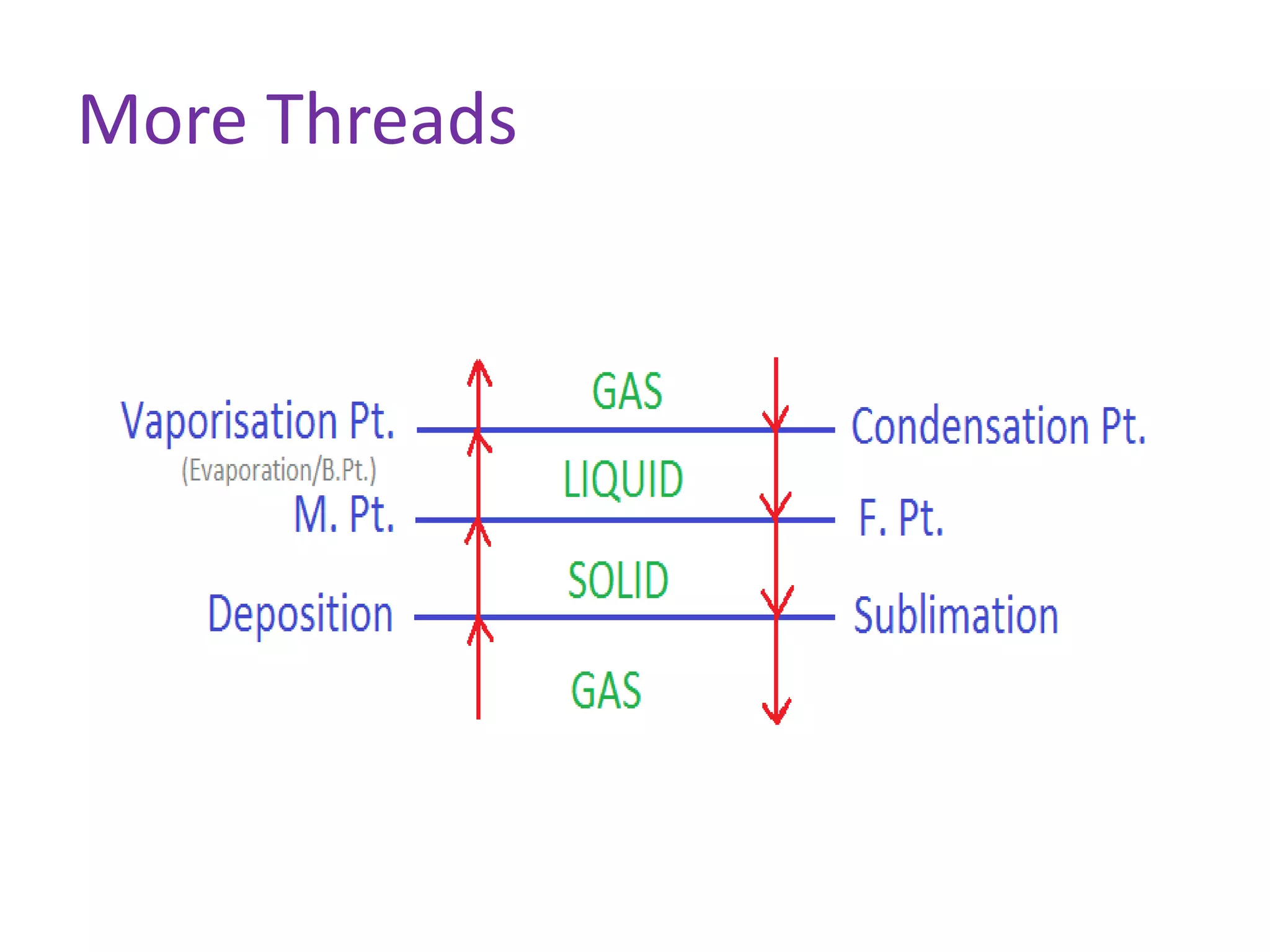
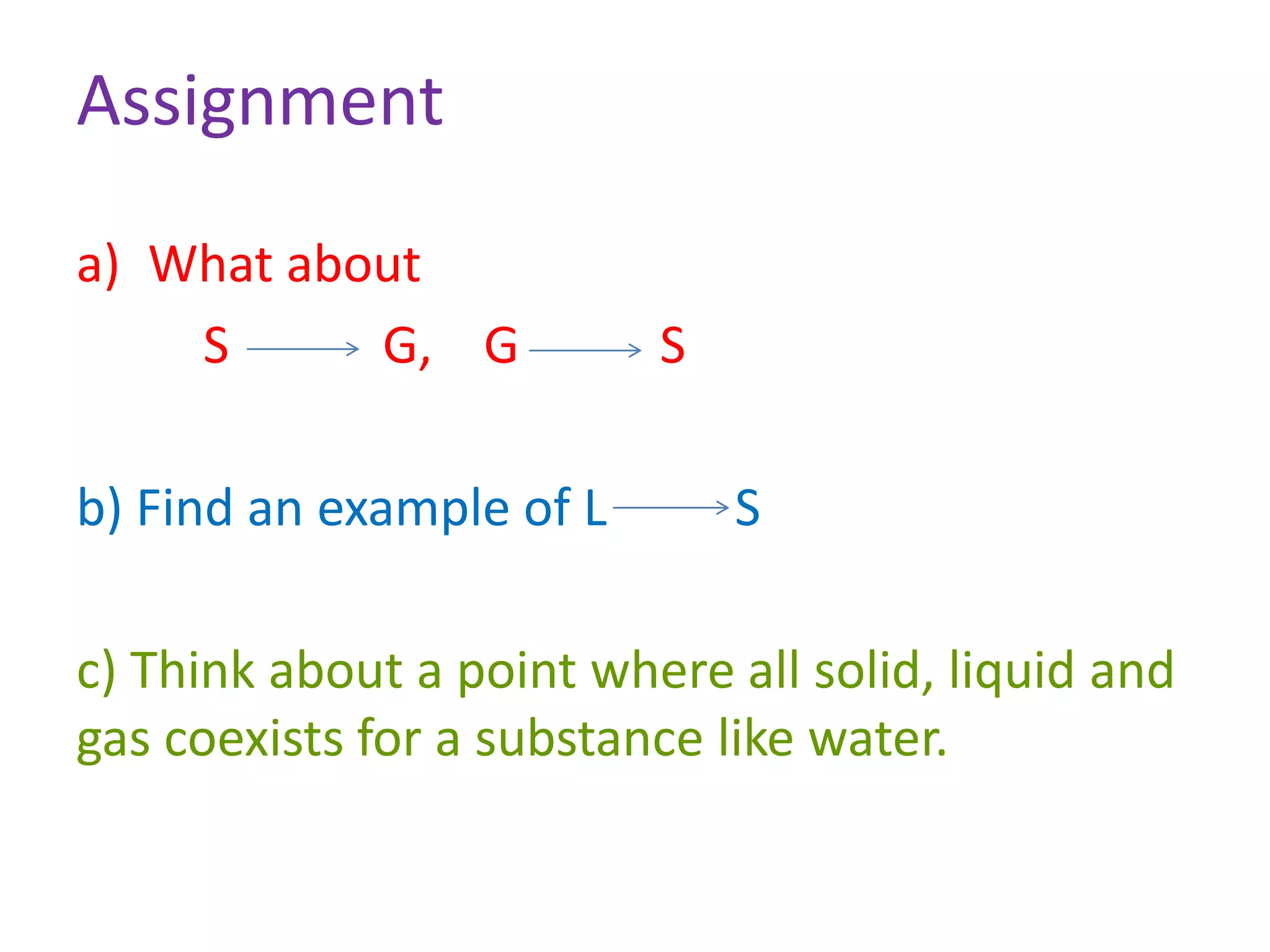


This document discusses state changes of matter under varying temperature and pressure conditions. It defines key transition temperatures like melting point, boiling point, and freezing point. These transition temperatures can be used to determine purity of substances. Knowledge of state changes is important for applications like natural thermostats, testing purity during transportation and ensuring safety. The document quizzes readers with multiple choice questions on concepts like the correct phase change sequence, definitions of transition temperatures, and using boiling point to determine purity.









