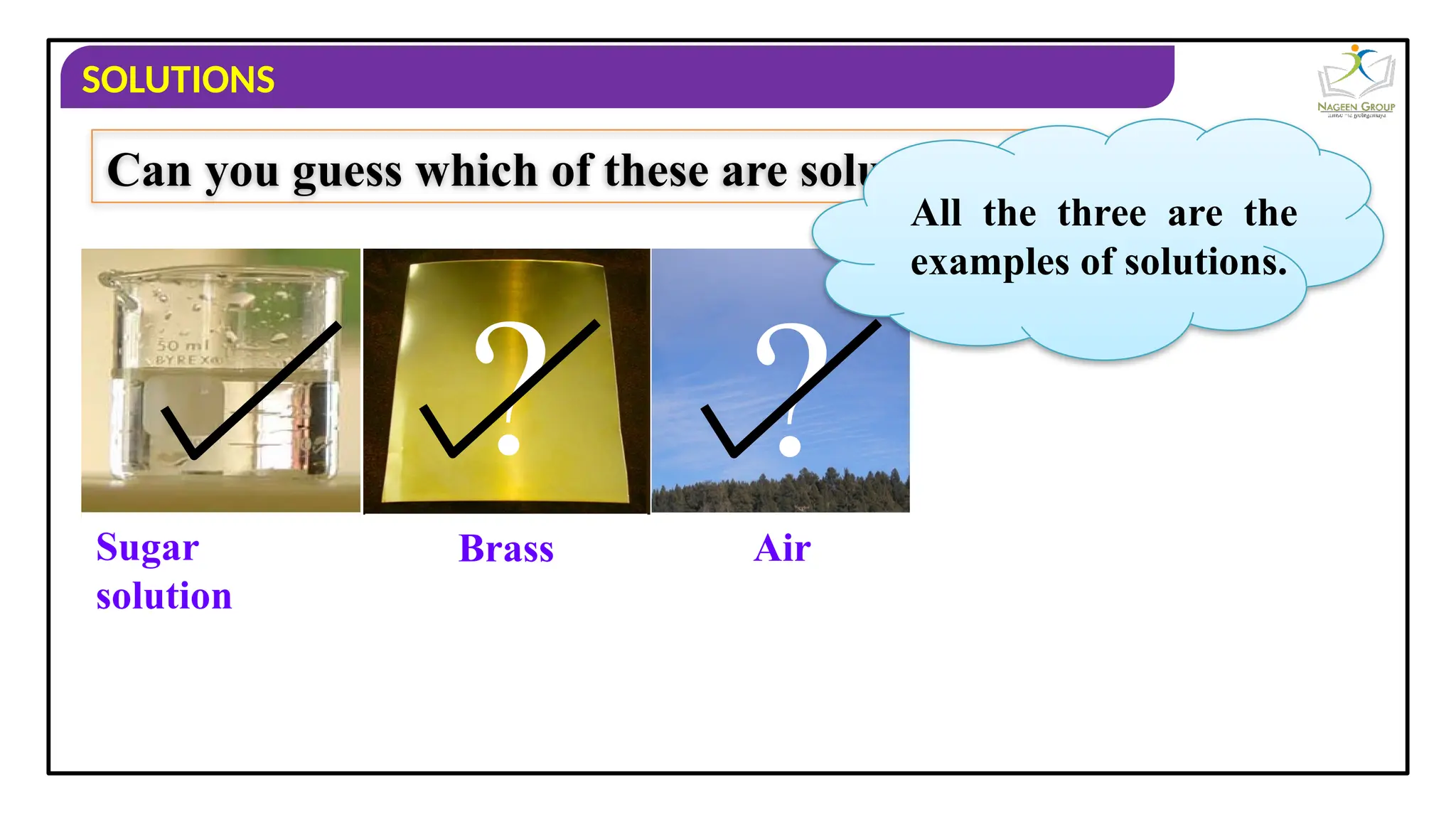
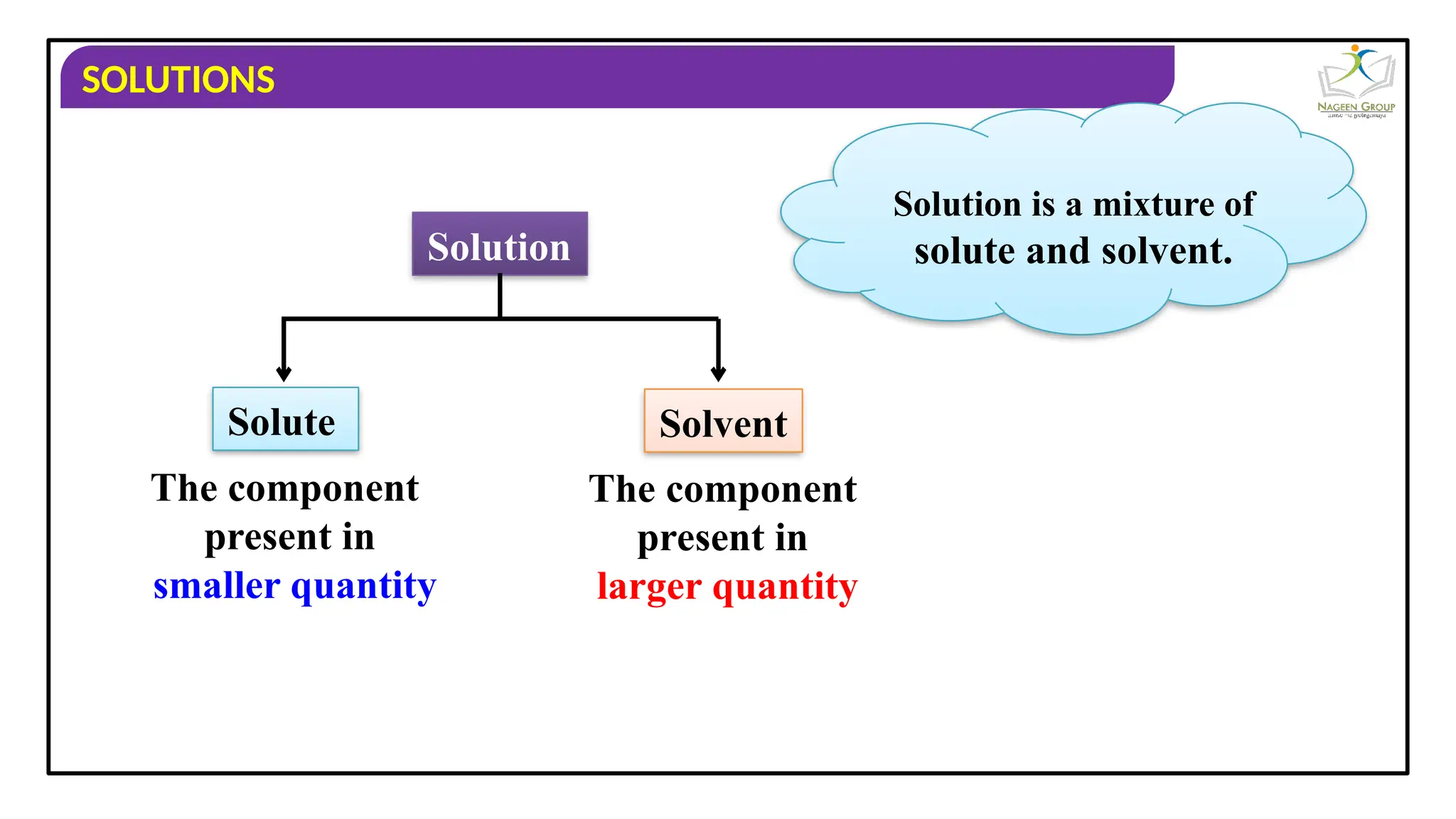
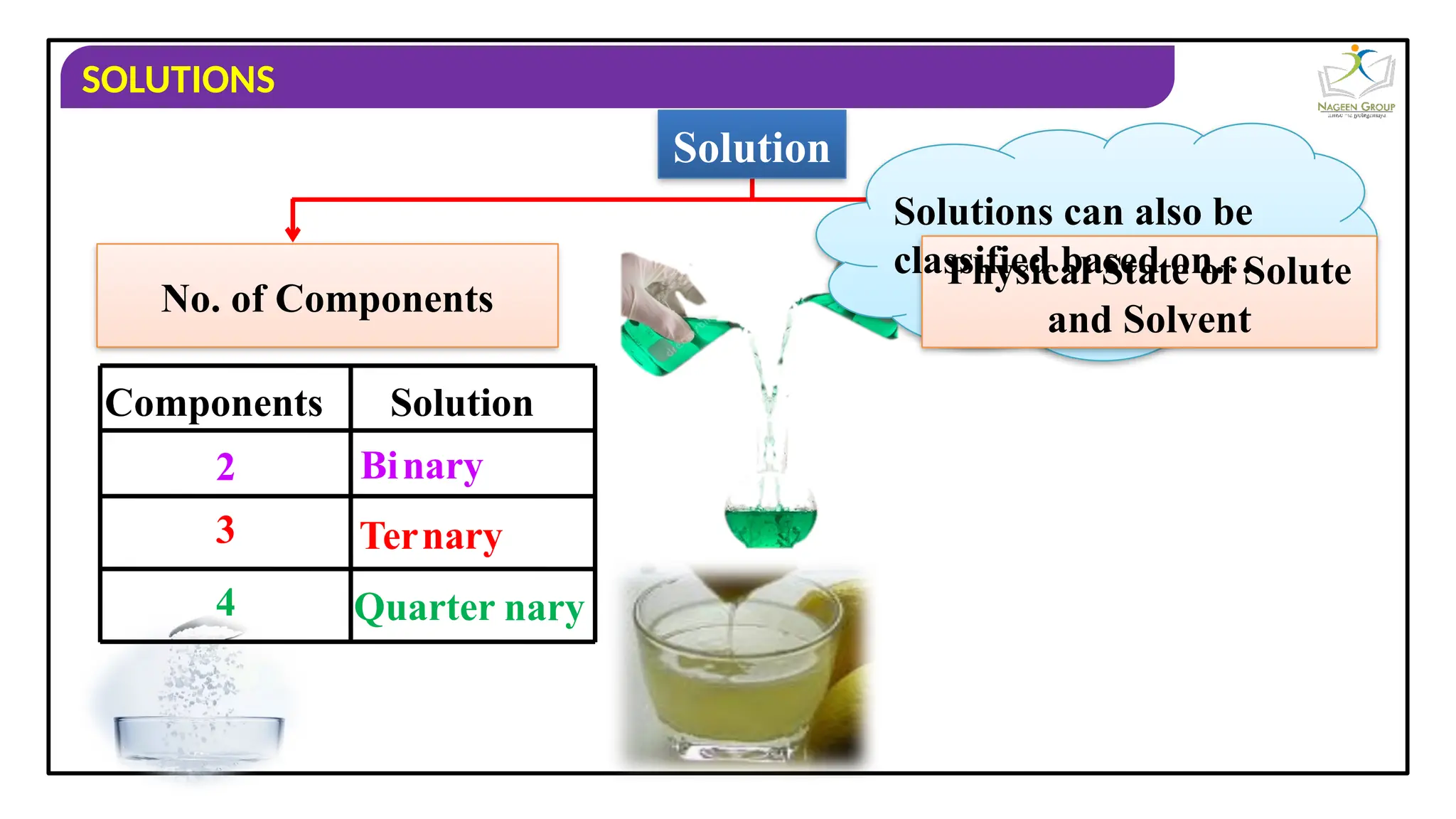
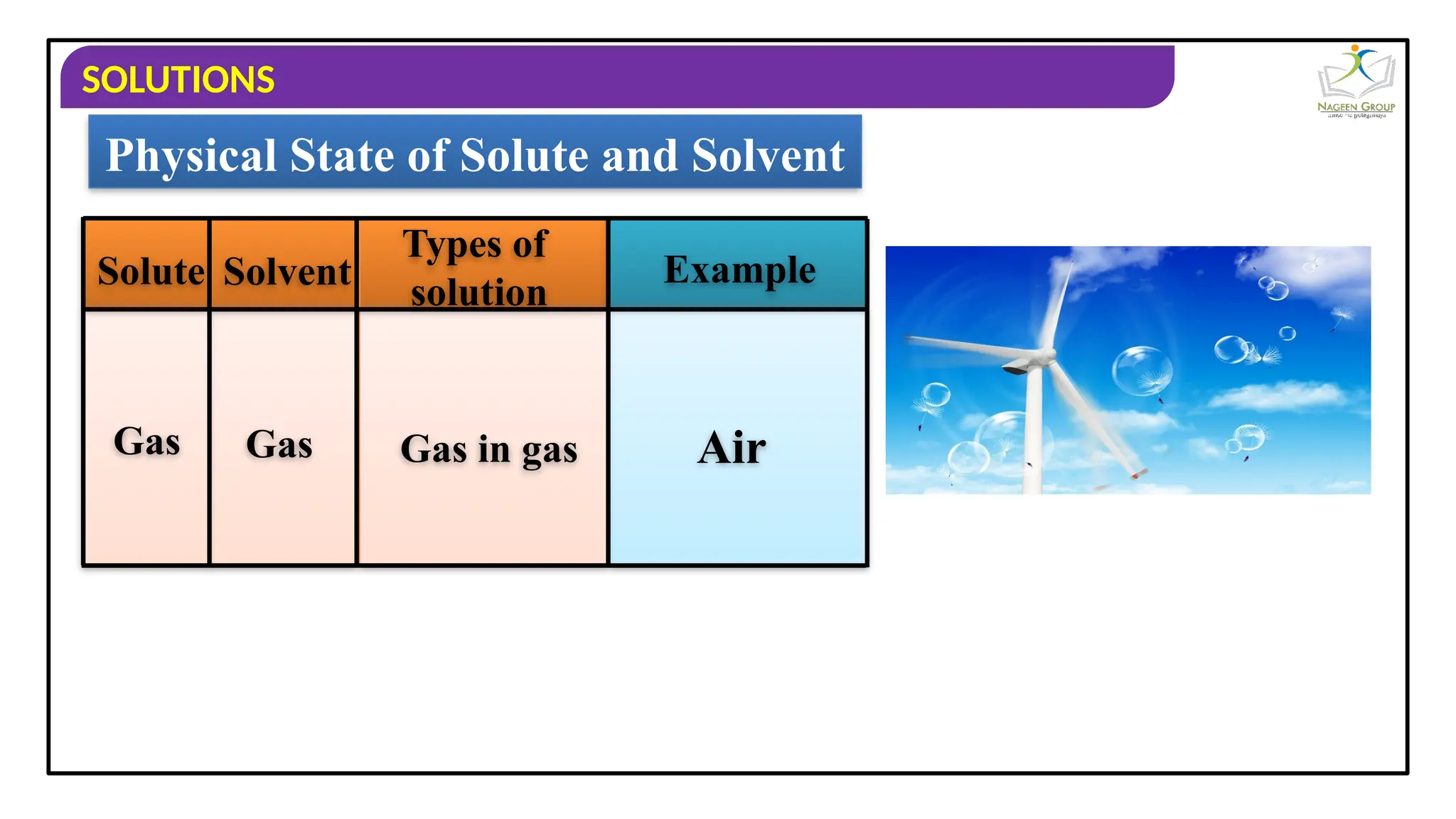
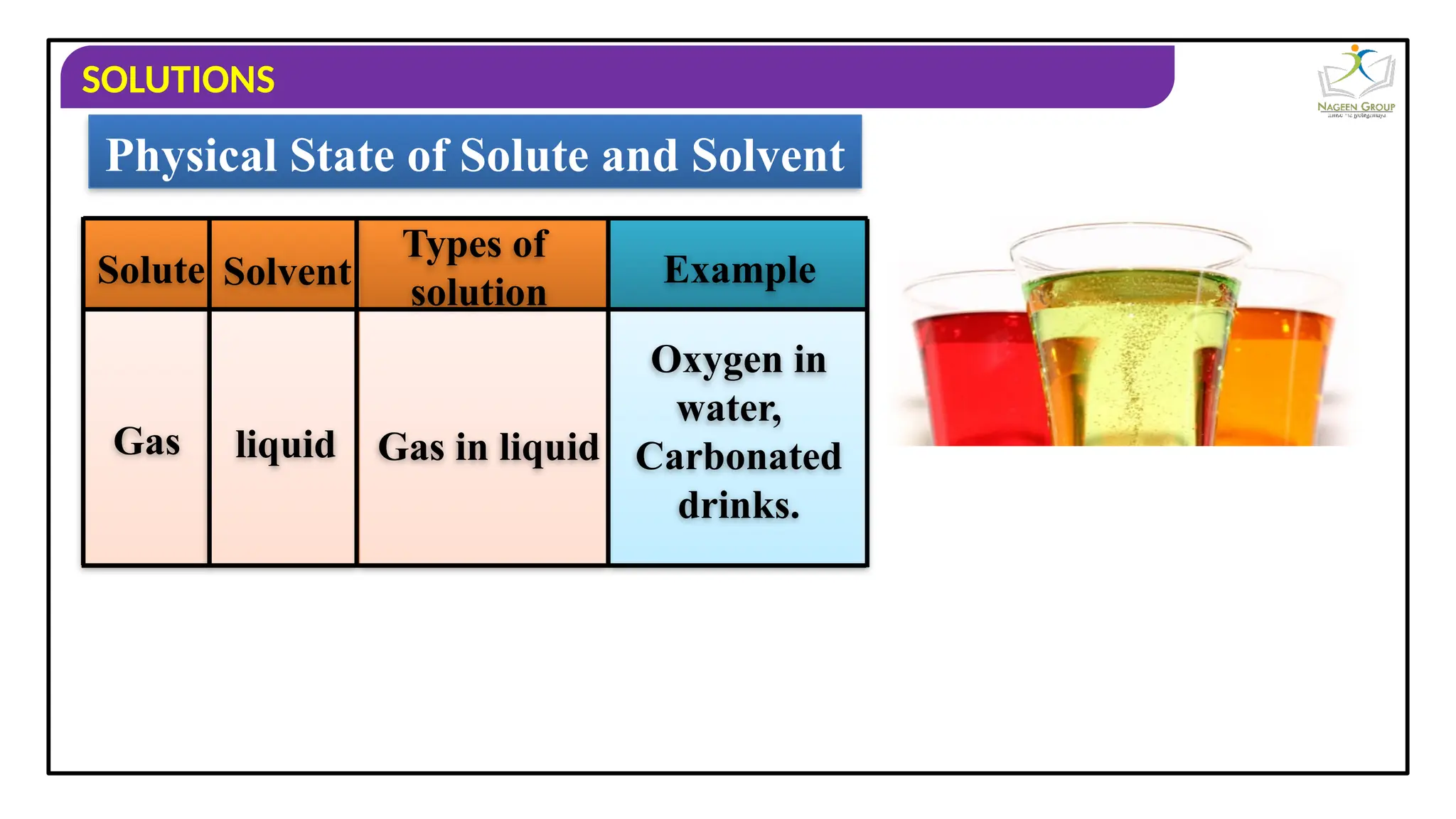
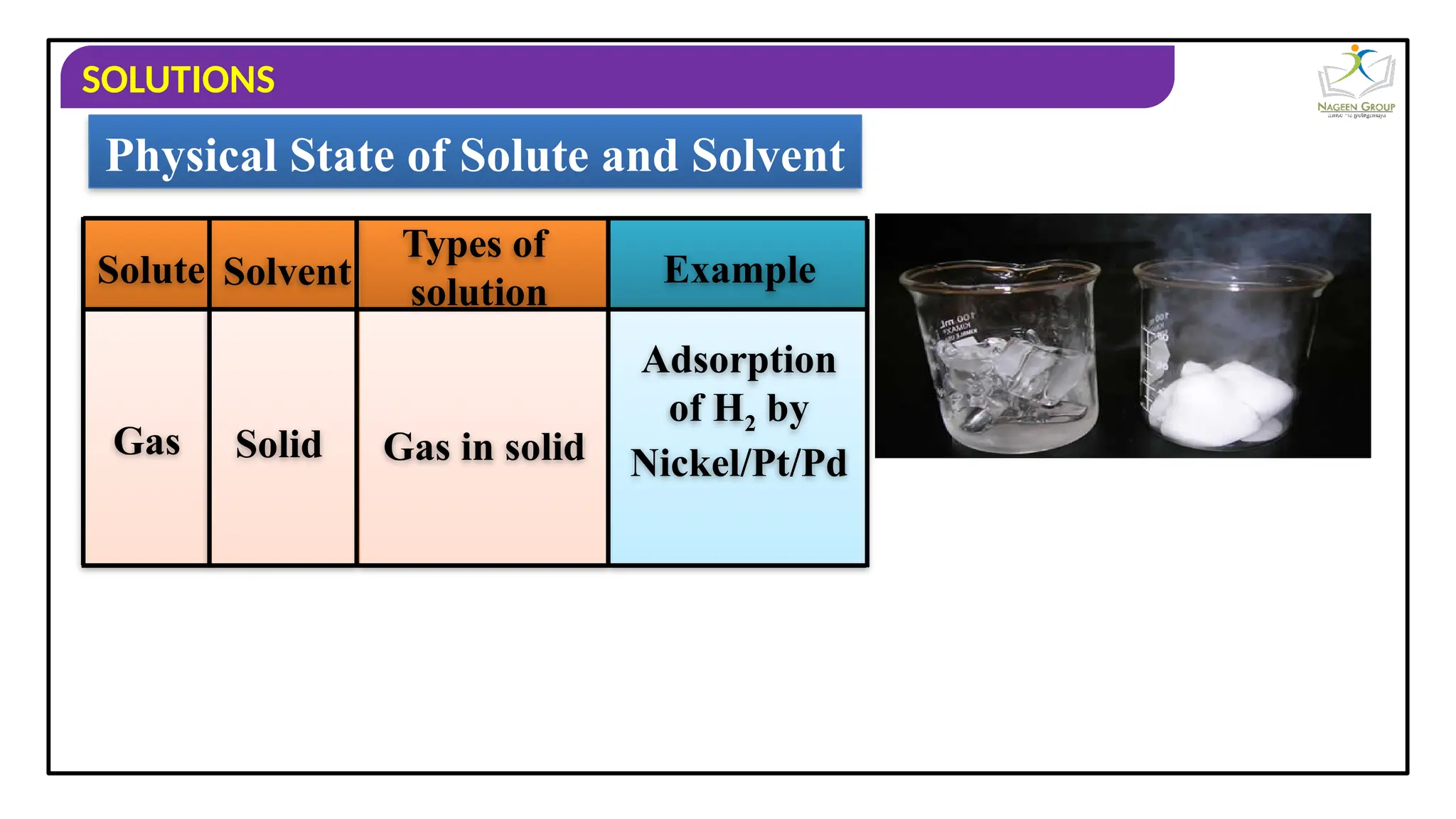
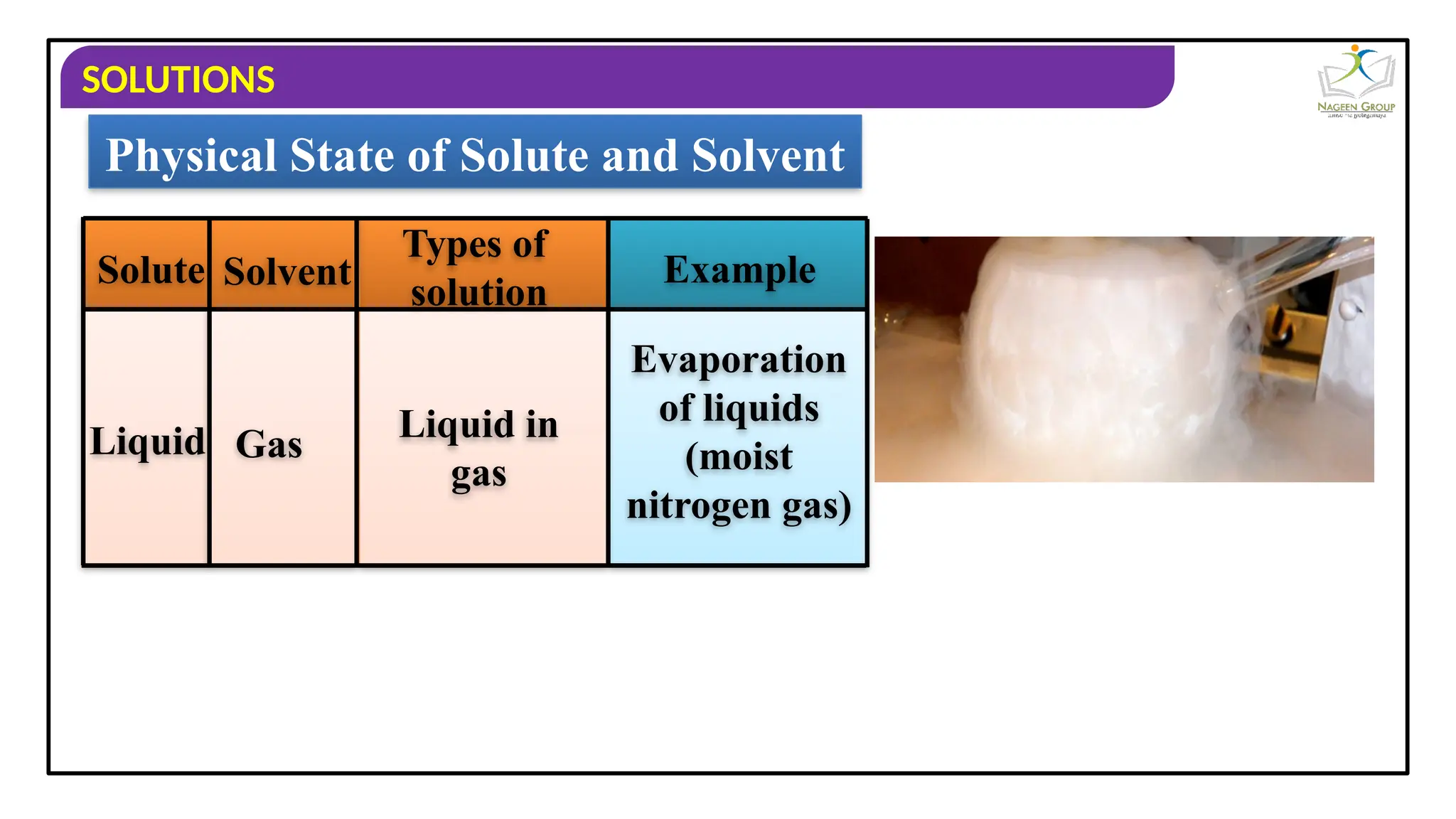
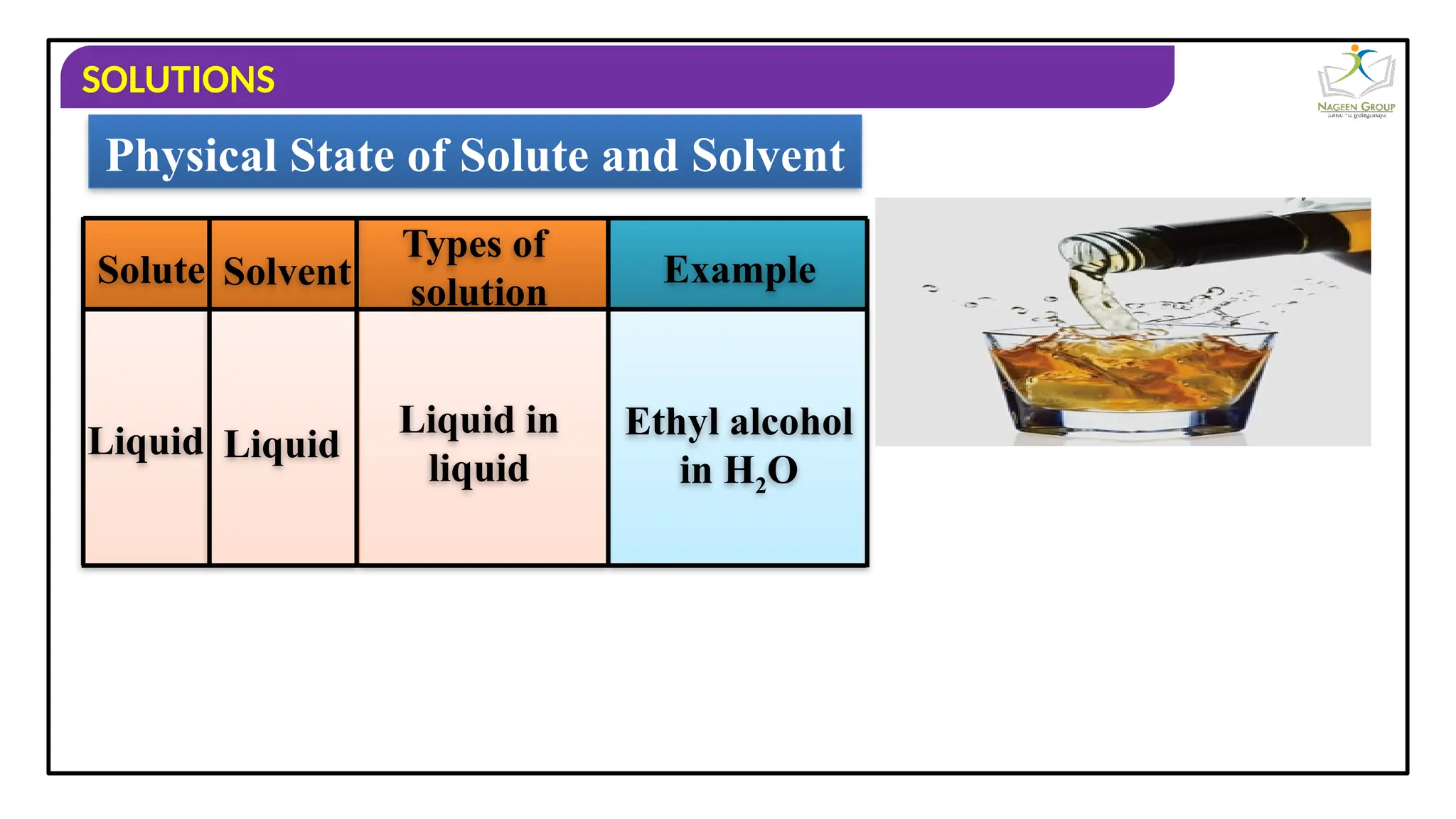
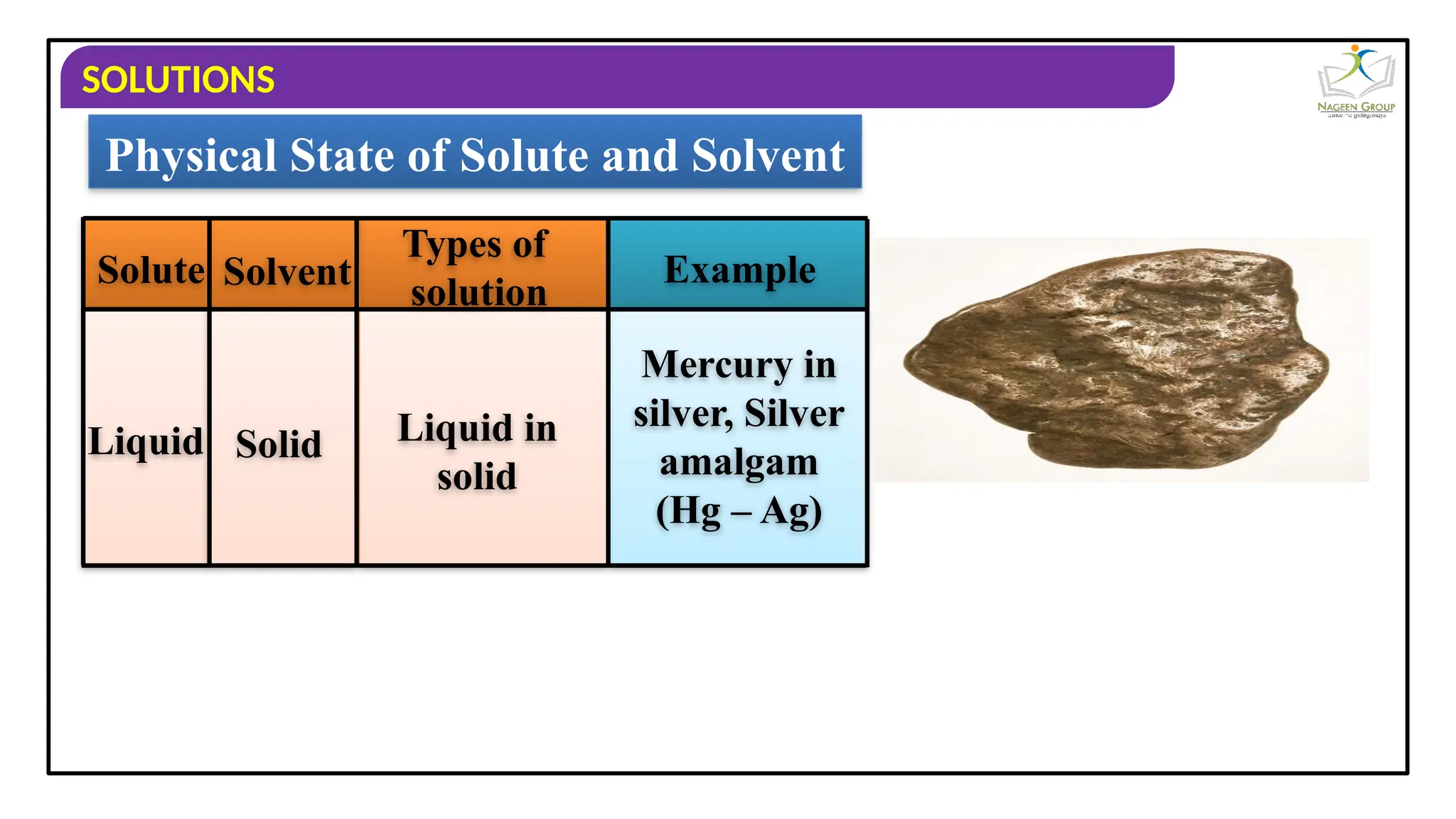
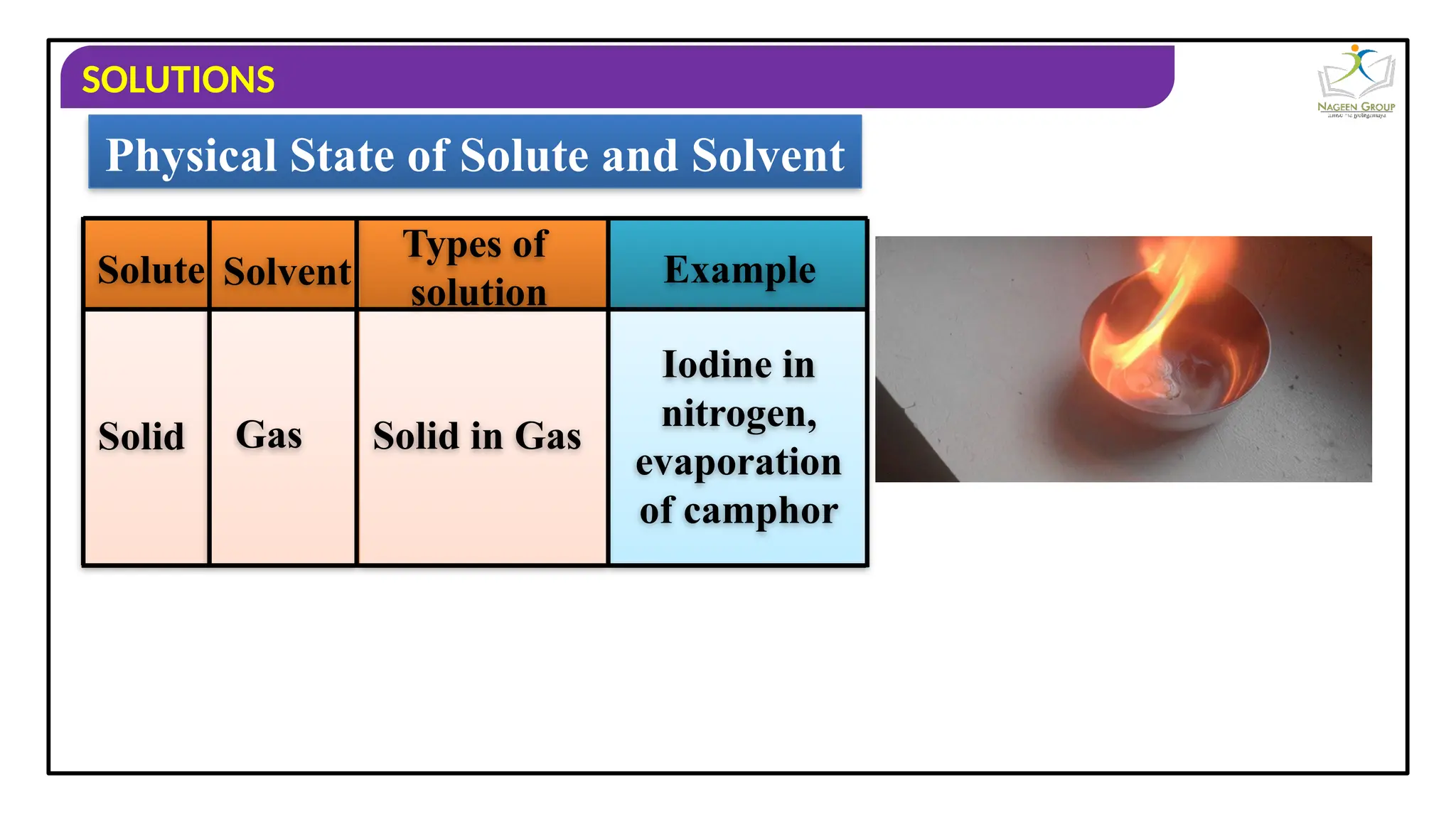
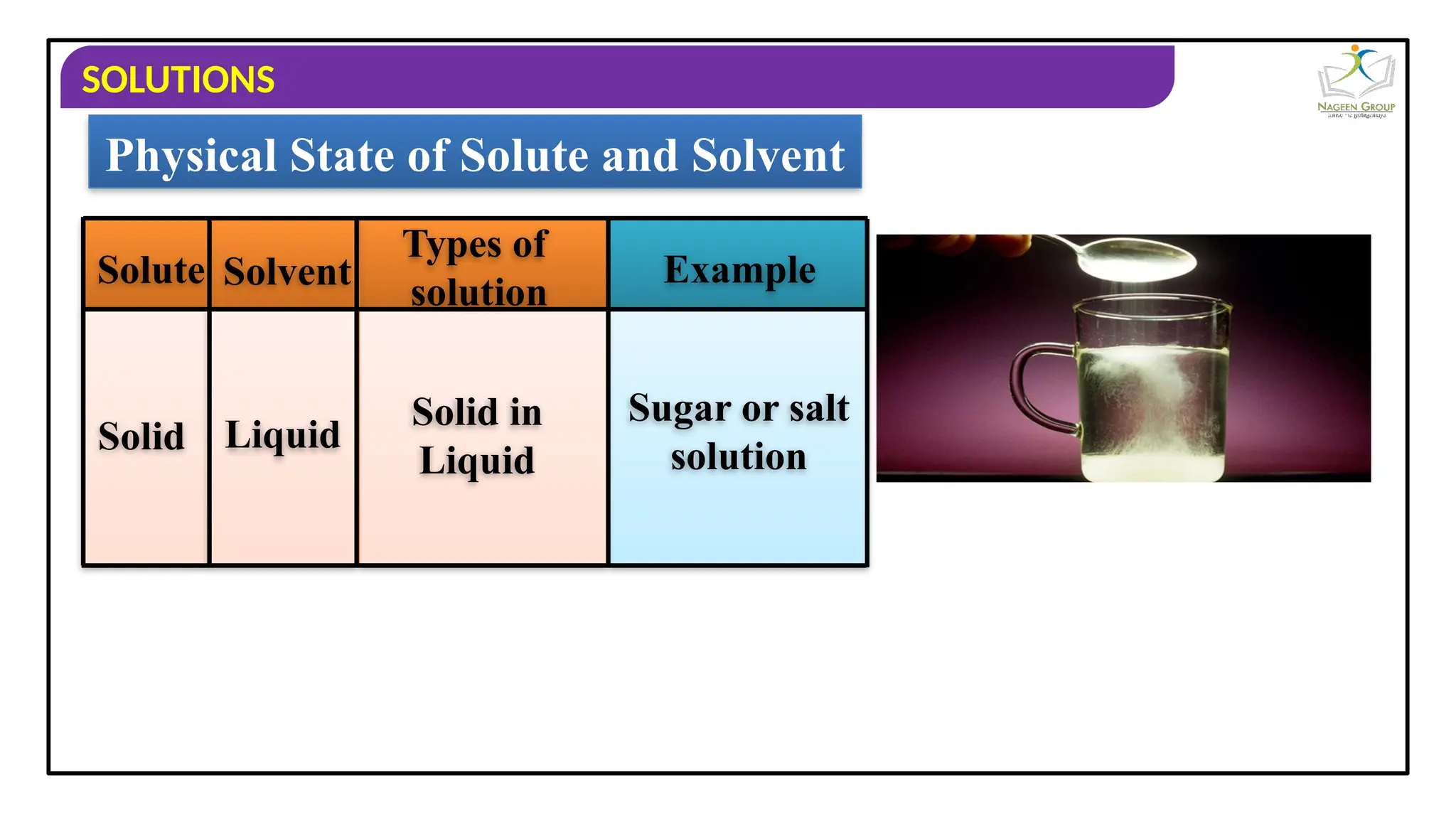
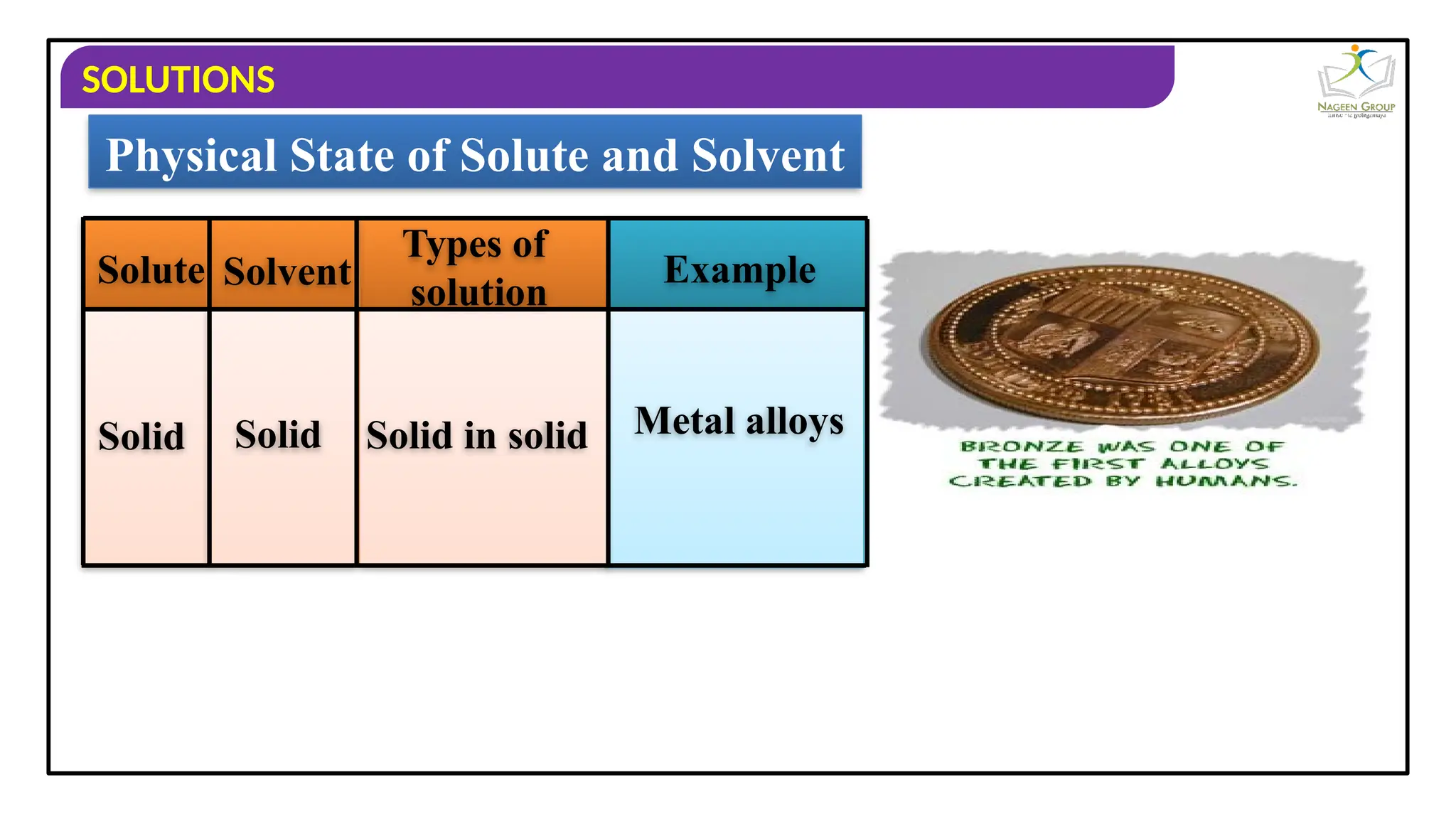
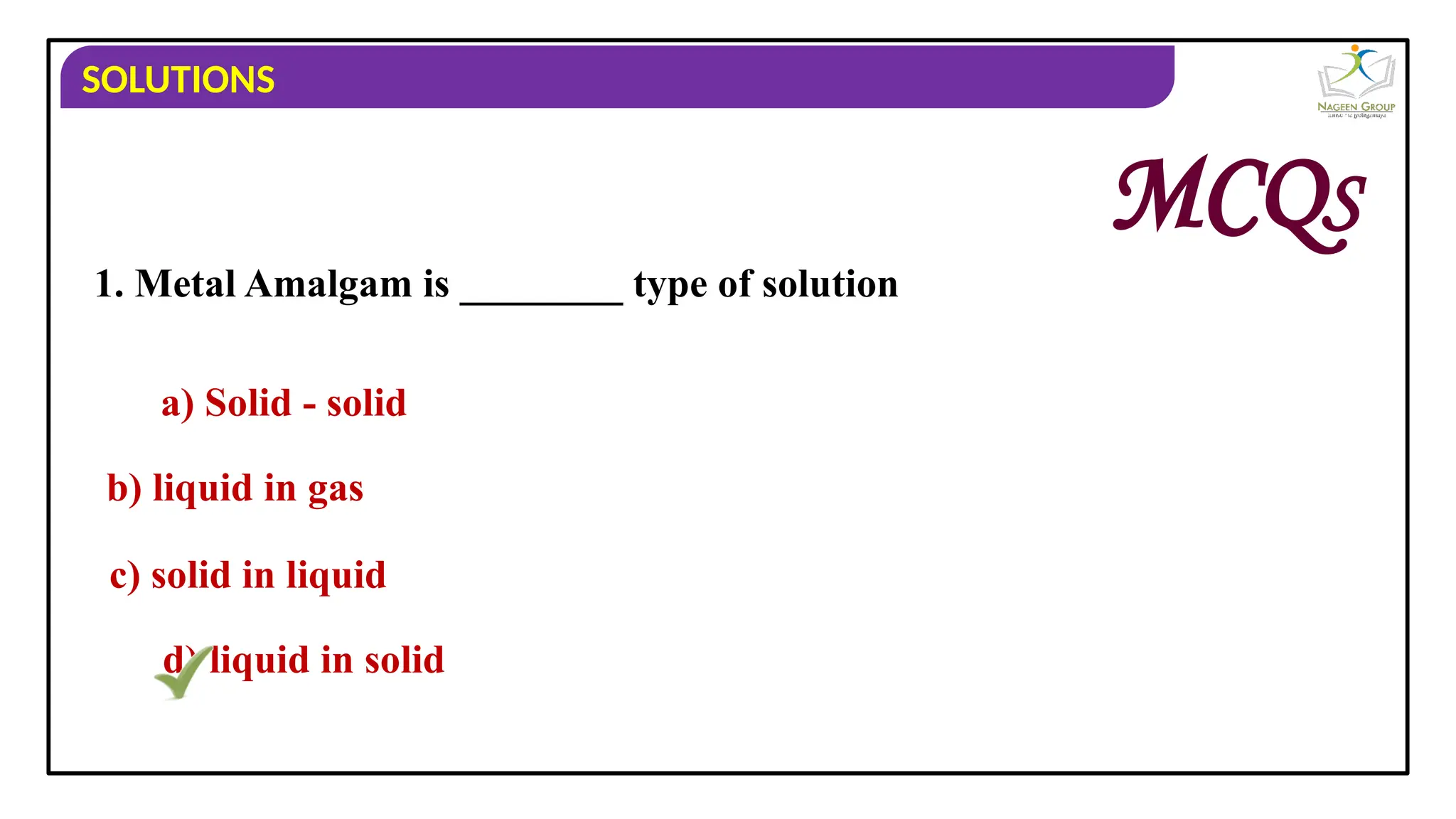
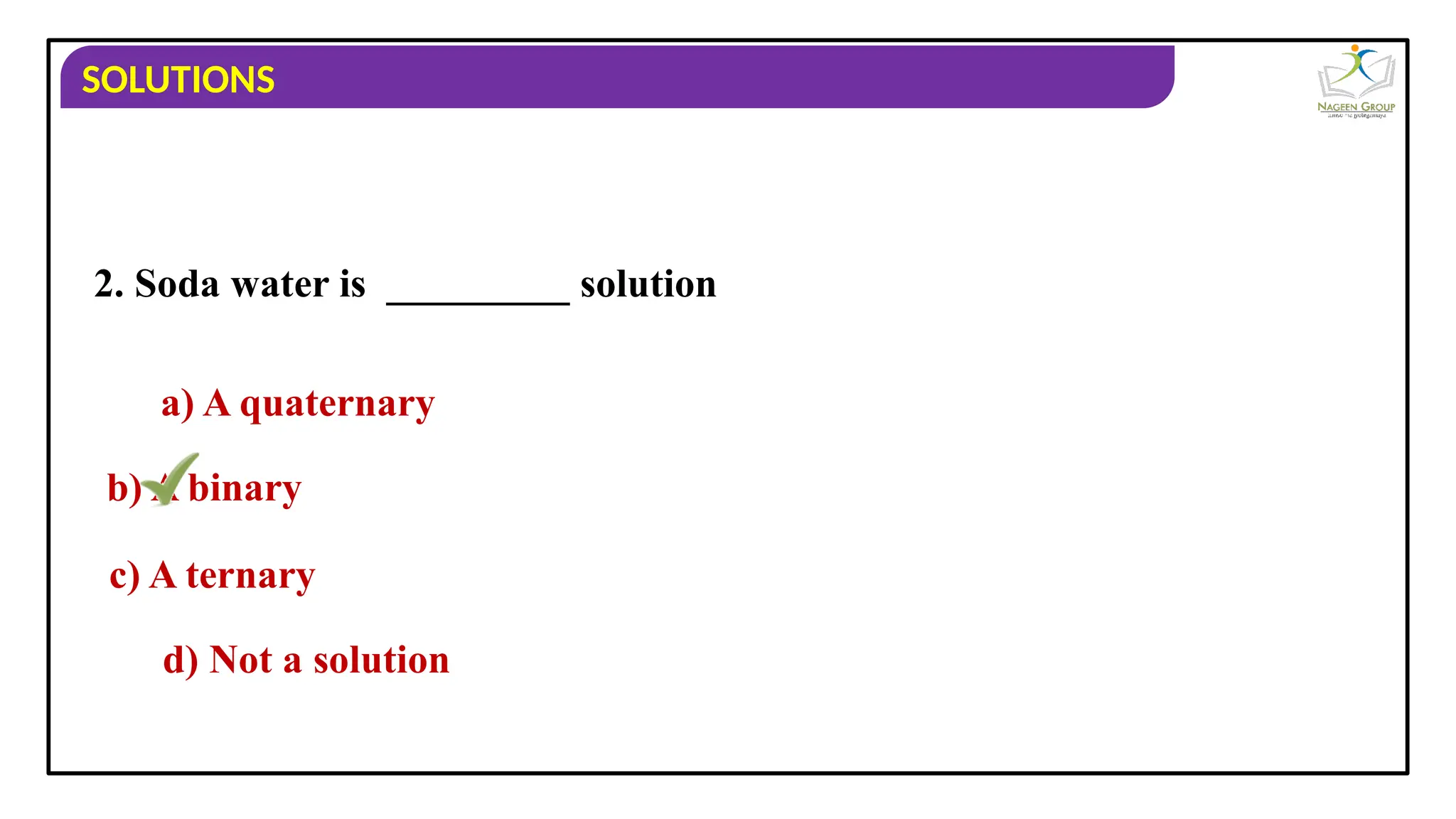
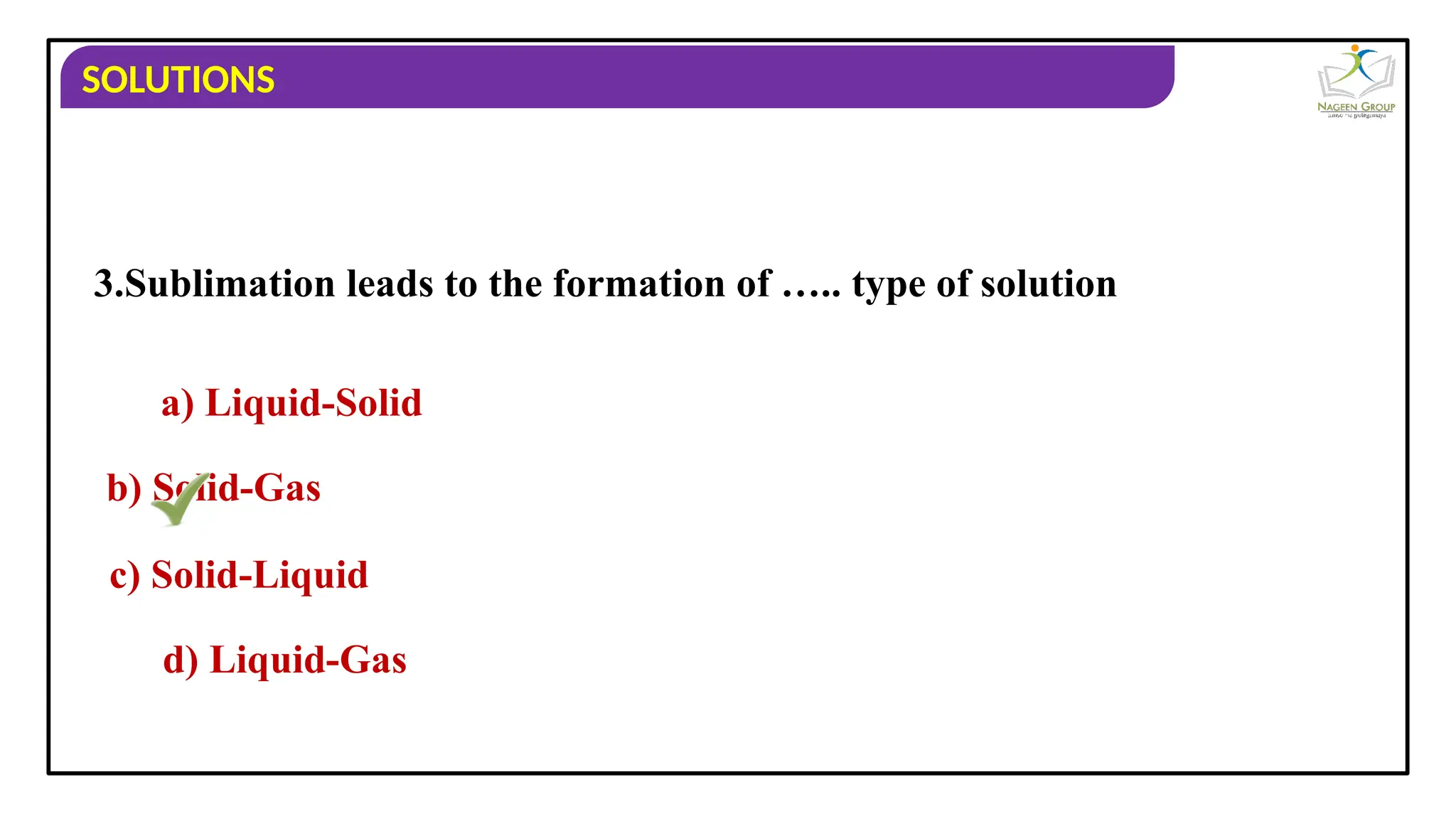
The document provides an overview of solutions, defining them as homogeneous mixtures of two or more components, which can be in solid, liquid, or gaseous states. It explains the roles of solute and solvent in solutions and discusses various types of solutions classified by the physical states of their components, including examples like soda water and brass. Additionally, it includes multiple-choice questions to reinforce understanding of the concepts presented.