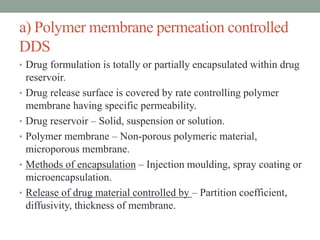
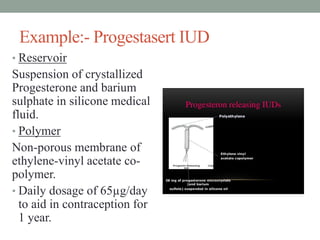
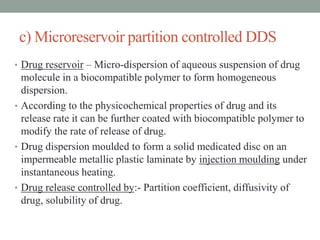
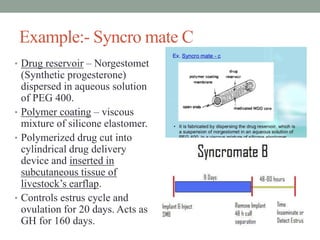
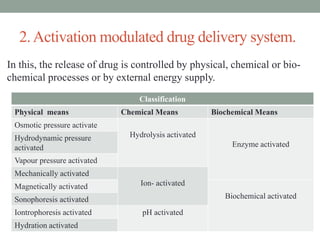
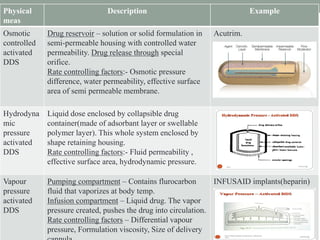
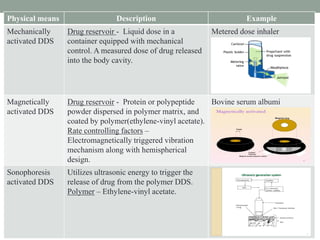
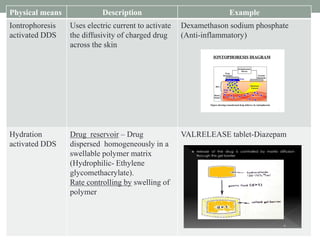
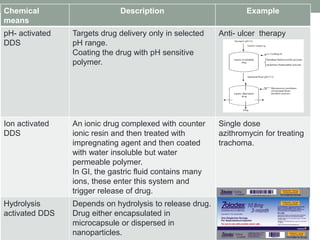
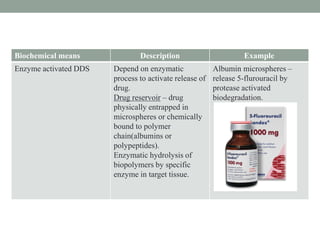
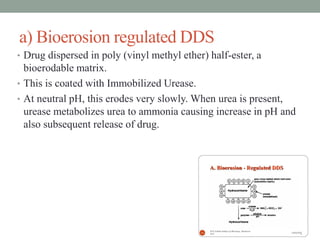
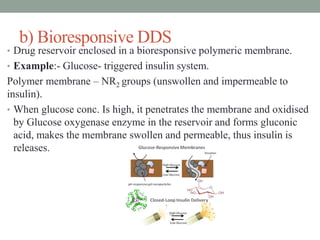
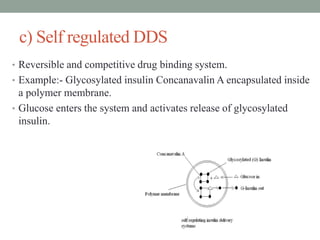
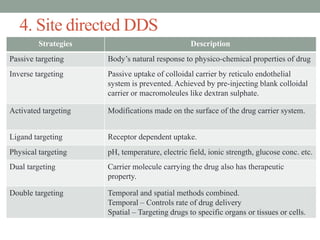
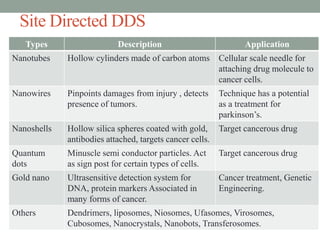
This document provides an overview of controlled drug delivery systems. It discusses sustained and controlled release, advantages, and classifications including rate pre-programmed, activation modulated, feedback regulated, and site targeting drug delivery systems. Specific examples are described for polymer membrane permeation controlled, polymer matrix diffusion controlled, and microreservoir partition controlled rate pre-programmed systems. Activation modulated systems can use physical, chemical, or biochemical means to control drug release. Feedback regulated systems respond to triggering substances to control bioerosion, bioresponses, or through self-regulation. Site targeting aims to direct drugs to specific organs, tissues, or cells either passively or through modifications like ligands.