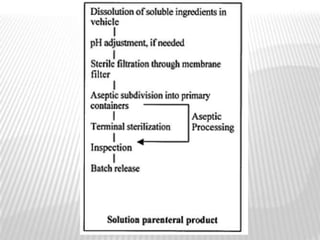
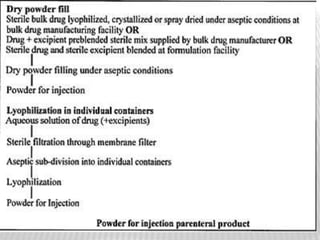
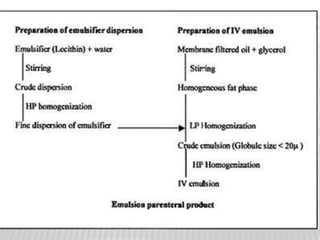
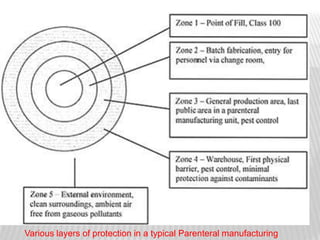
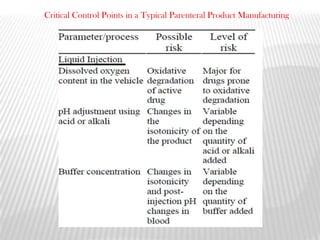
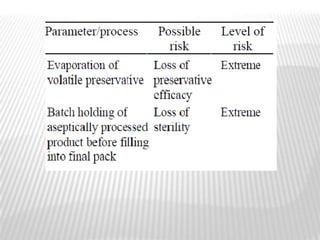
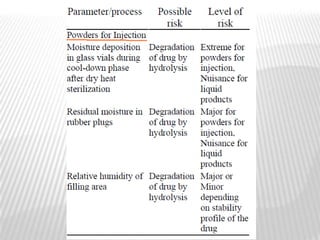
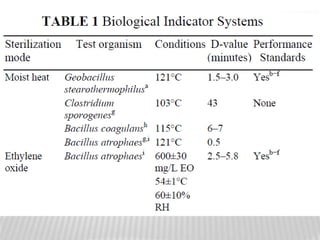
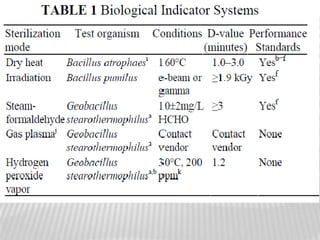
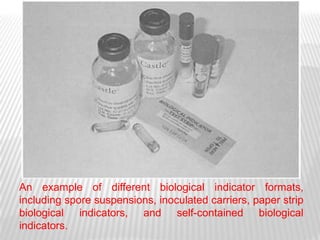
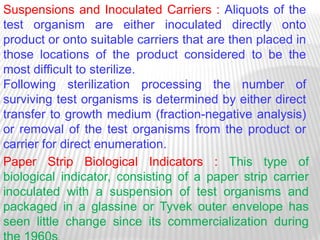
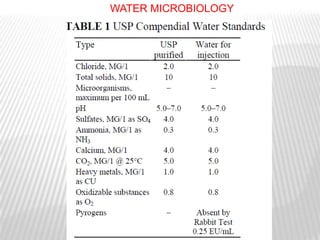
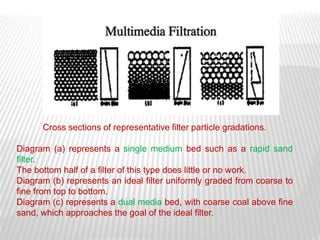
This document discusses microbial contamination control in parenteral manufacturing. It outlines various layers of protection used, including terminal sterilization techniques like autoclaving. It also discusses aseptic processing and sources of contamination control strategies during aseptic manufacture. Other topics covered include blow-fill-seal technology, issues in sterilization by filtration, sterile prefilled syringes, process validation, hazard analysis and critical control points. Key sterilization techniques and the selection of appropriate test organisms to validate these processes are also summarized.