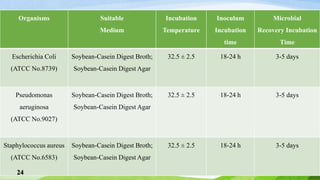
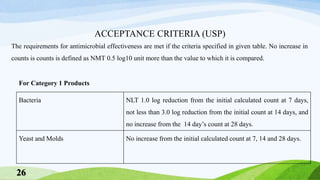
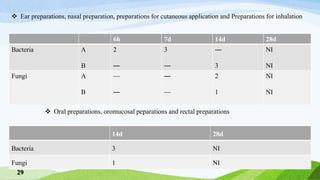
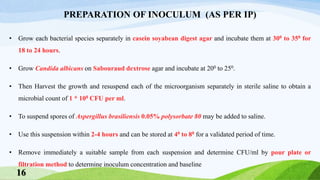
The document outlines antimicrobial effectiveness testing for pharmaceutical products, emphasizing the need for preservatives in both sterile and non-sterile formulations to inhibit microbial growth. It details testing procedures, required microorganisms, and acceptance criteria for determining the efficacy of antimicrobial agents in various product categories, including injections, topical applications, and oral preparations. The information presented is crucial for ensuring product safety and compliance with pharmacopoeial standards.



















![PROCEDURE (AS PER USP)
The procedure can be conducted either in five original containers if sufficient volume of product is available in
each container and the product container can be entered aseptically (i.e., needle and syringe through an elastomeric
rubber stopper),or in five sterile caped bacteriological containers[inert relative to antimicrobial agents ] of suitable
size into which a sufficient volume of product has been transferred.
Inoculate each container with one of the prepared and standardized inocula, and mix. The volume of suspension
inoculum used is between 0.5% and 1.0% of the volume of the product to minimize potential effects on the
product.
The concentration of viable microorganisms that is added to the product (Category 1,2,3) is such that the final
concentration of test preparation after incubation is between 1x105and 1x106 cfu/ml of product.
For category 4 products (antacids) final concentration of test preparation is 1x103 and 1x104 cfu/ml of product.
22](https://image.slidesharecdn.com/3-240104075731-69caf662/85/3-Antimicrobial-Effectiveness-Final-pptx-22-320.jpg)