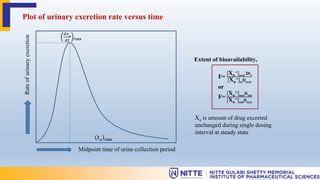
Bioavailability is defined as the rate and extent of absorption of a drug from its dosage form. It is determined by comparing the absorption of a drug formulation to a reference standard, usually intravenous administration. There are two main categories for quantitatively measuring bioavailability - pharmacokinetic and pharmacodynamic methods. Pharmacokinetic methods, like plasma level-time studies, are most widely used and reliable. They involve collecting serial blood samples after drug administration to obtain plasma concentration-time profiles and calculate parameters like Cmax, Tmax, and AUC. Urinary excretion studies can also be used if a significant portion of the drug is excreted unchanged in urine. Pharmacodynamic methods measure physiological effects but are more variable and difficult to