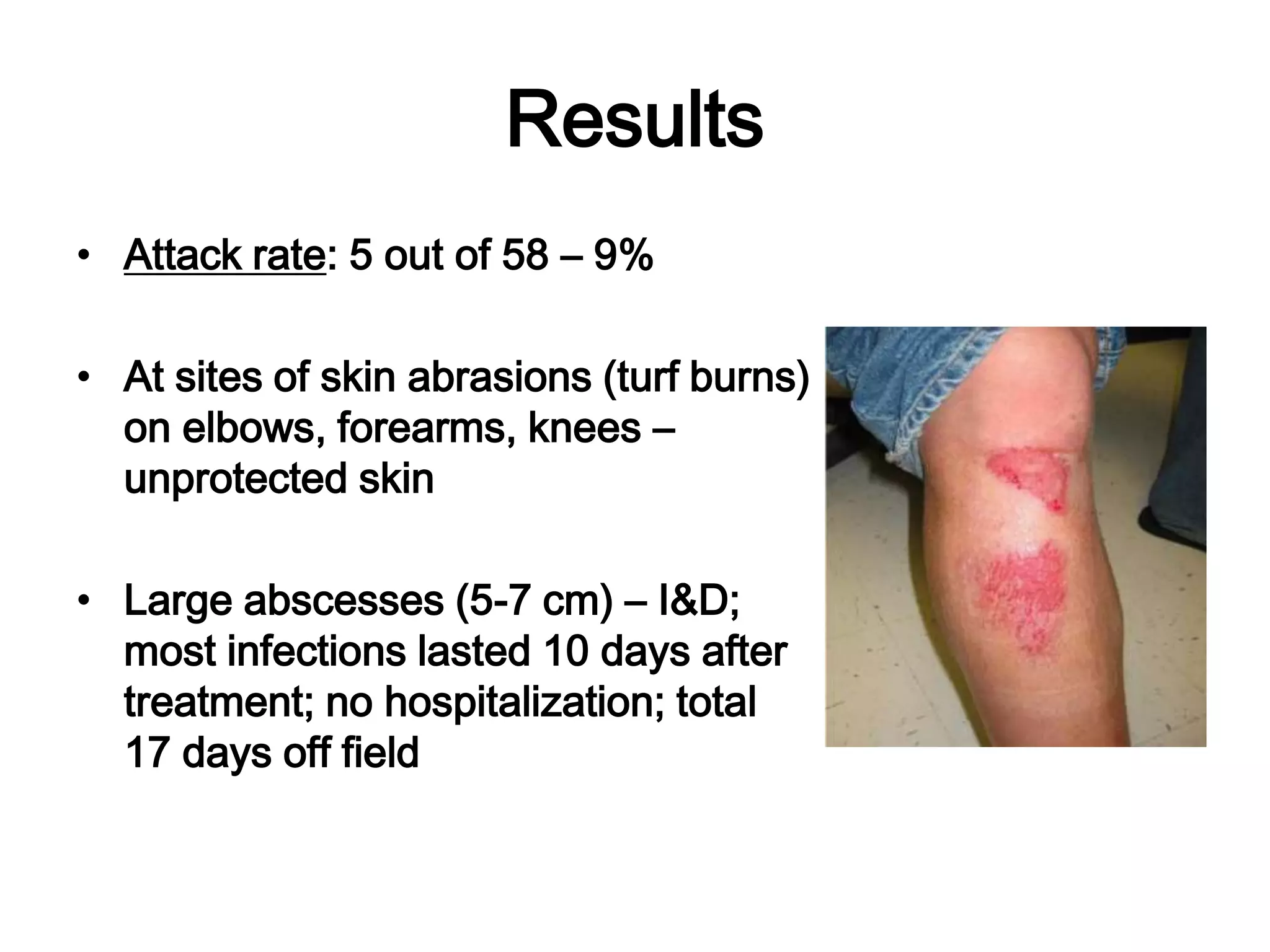
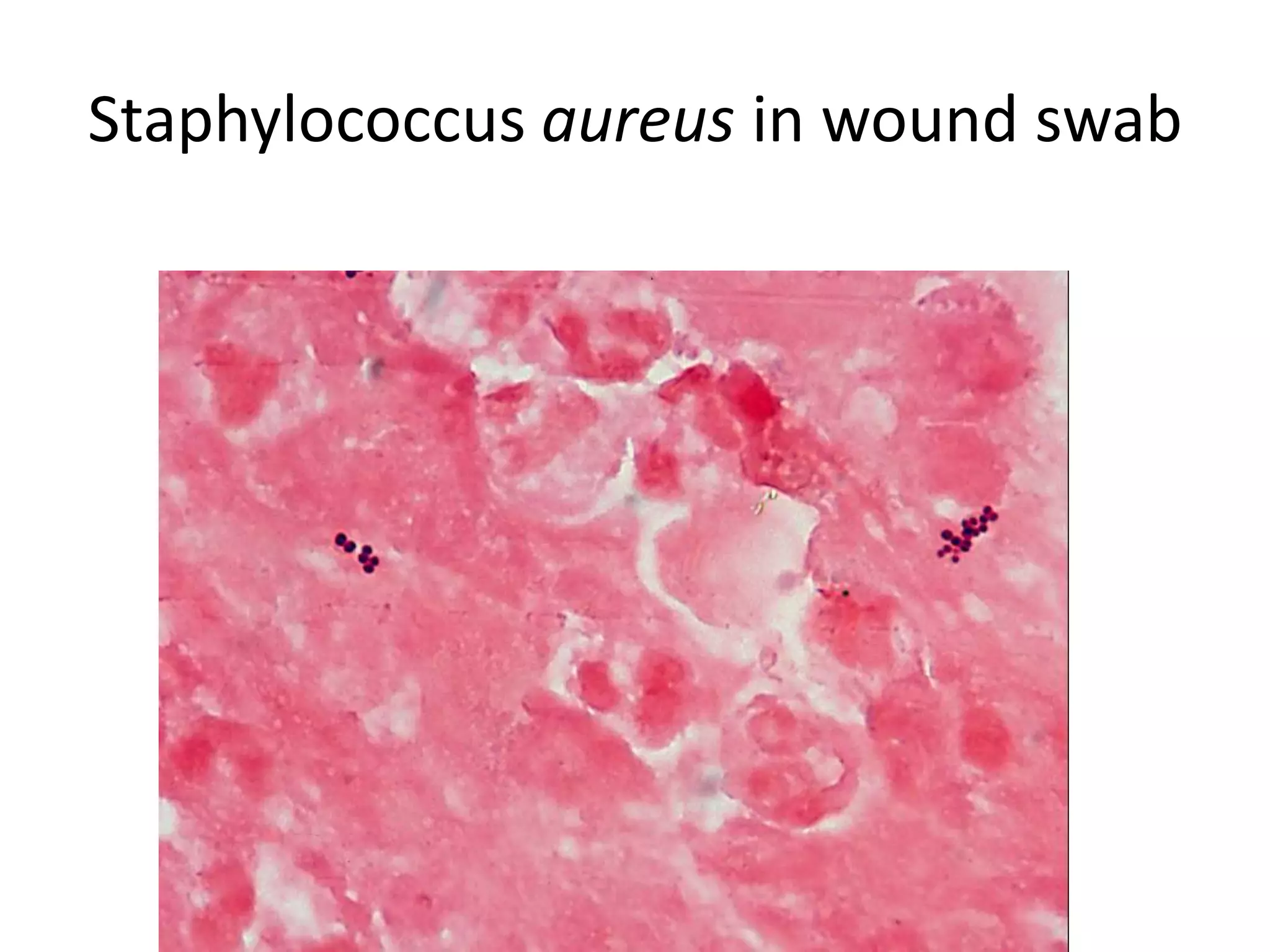
This document discusses Methicillin Resistant Staphylococcus aureus (MRSA). It begins by describing the characteristics of Staphylococcus including that it is a gram-positive coccus that can cause a variety of infections in humans. It then discusses the evolution of antibiotic resistance in S. aureus from penicillin to methicillin to vancomycin. It also covers the differences between hospital-acquired MRSA versus community-acquired MRSA and risks for infection. Treatment options for skin infections caused by MRSA are also summarized.













![Evolution of Resistance in S. aureus
Penicillin Methicillin
Penicillin-resistant Methicillin-
S. aureus resistant
[1950s] S. aureus [1970s]
S. aureus (MRSA)
Vancomycin
[1997]
[1990s]
Vancomycin Vancomycin Vancomycin-resistant
resistant [ 2002 ] intermediate- enterococci (VRE)
S. aureus resistant
S. aureus
(VISA)](https://image.slidesharecdn.com/methicillinresistantstaphylococcusaureus-120403194010-phpapp02/75/Methicillin-resistant-staphylococcus-aureus-15-2048.jpg)