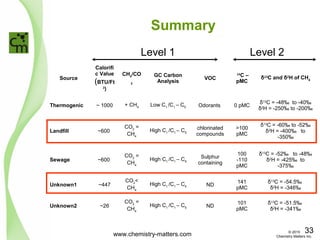
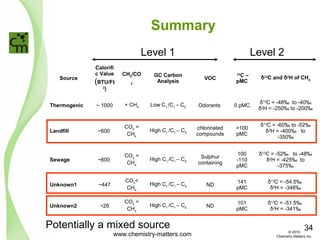
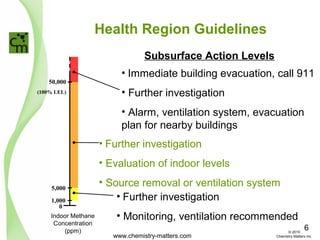
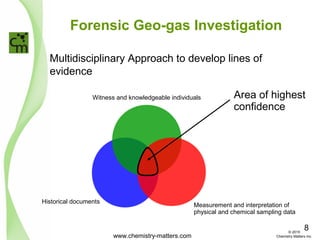
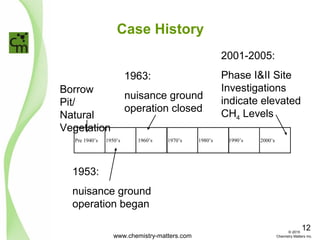
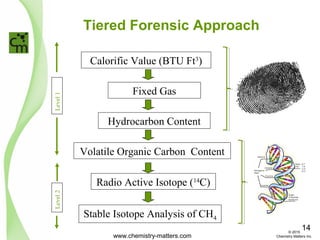
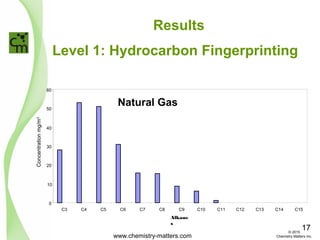
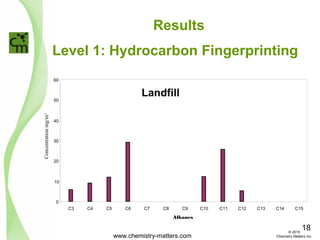
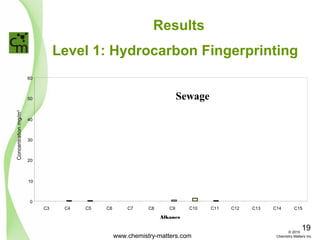
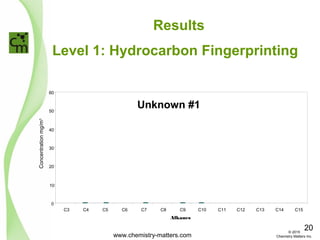
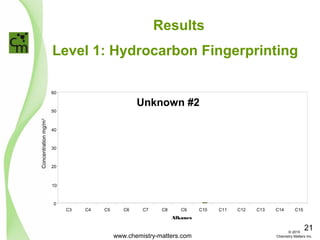
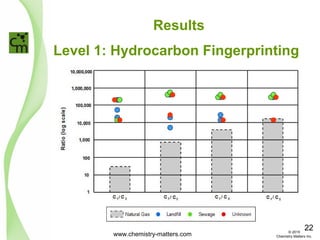
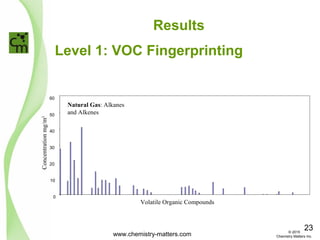
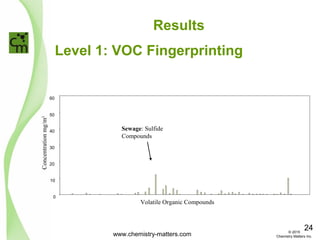
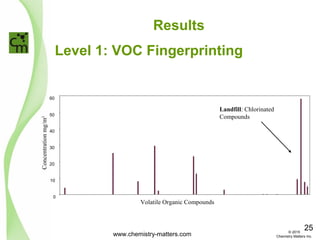
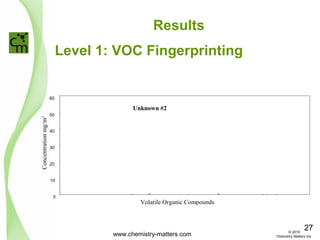
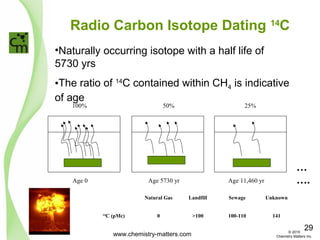
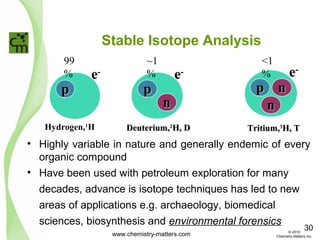
The document discusses using forensic techniques to identify sources of fugitive methane found in subsurface soils in a municipality. Level 1 analysis using fixed gas composition, hydrocarbon fingerprinting, and VOC analysis ruled out thermogenic sources but could not differentiate between landfill and sewage sources. Level 2 analysis using carbon dating, and stable isotope analysis of methane indicated one sampling point was likely degrading landfill material and another was a mixed source of landfill and organic soils. The analysis identified potential methane sources to help the client determine appropriate actions.











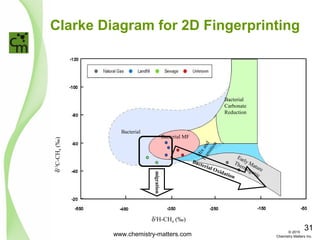
![Combination of Techniques
δ13
C-CH4 (‰)
C1/[C2+C3]
Sewage
Plant
Sewage
Plant
Unknown
Unknown
Landfill
Landfill
Historic
Landfill
Natural Gas
Natural Gas
Bacterial consumption of Methane will cause a
reduction in Methane concentration and
isotopic shift
Migration will cause a change in methane
concentration but not a large isotopic shift
Migration
Migration
Oxidation
www.chemistry-matters.com
32© 2015
Chemistry Matters Inc.](https://image.slidesharecdn.com/awma-2010-methane-forensics-presentation-trium-1277435581-phpapp01/85/Methane-forensics-techniques-for-source-allocation-32-320.jpg)