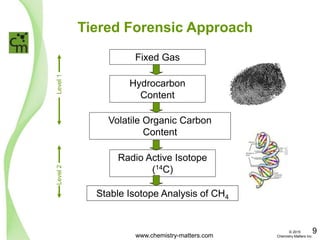
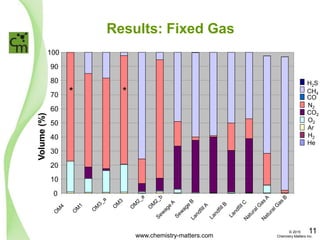
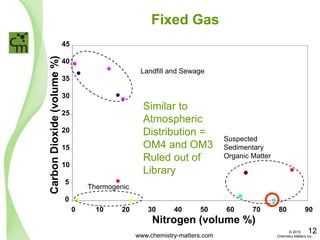
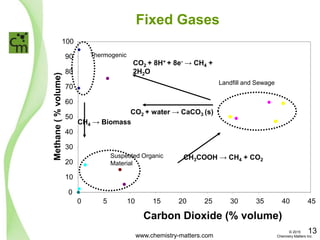
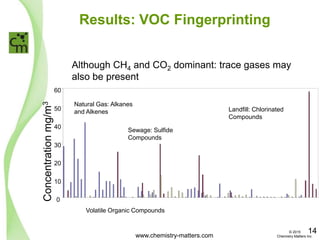
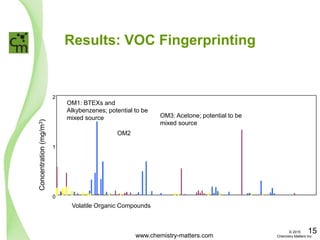
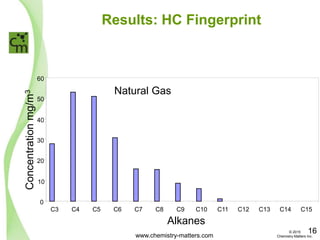
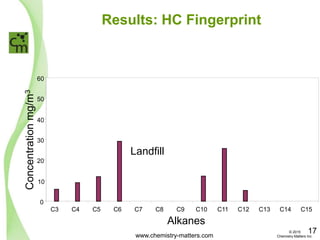
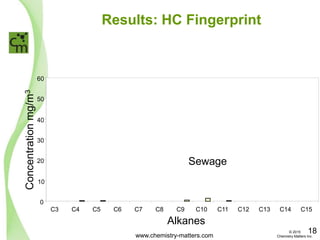
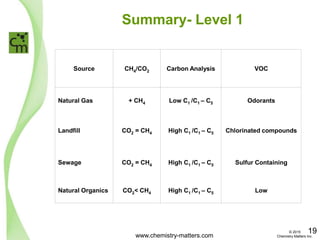
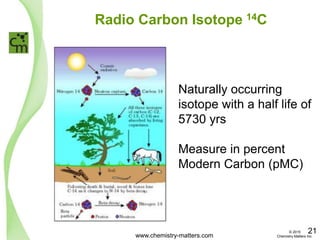
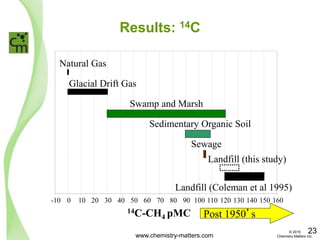
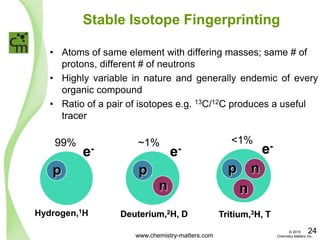
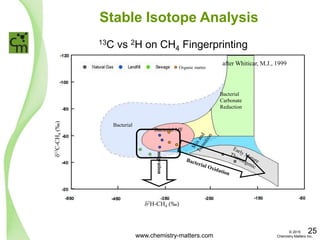
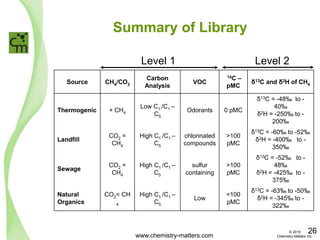
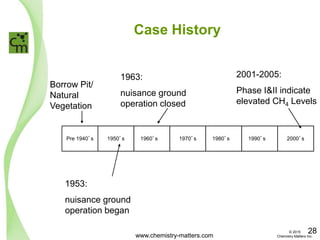
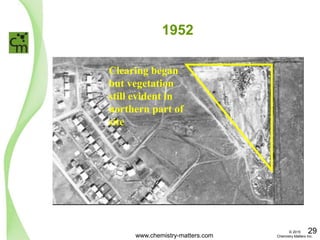
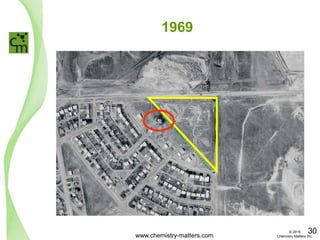
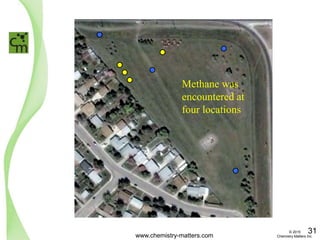
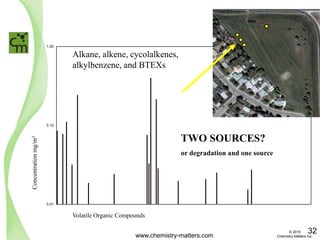
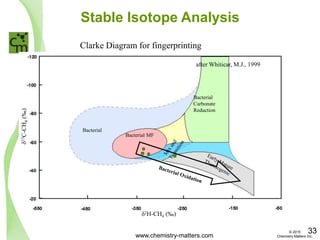
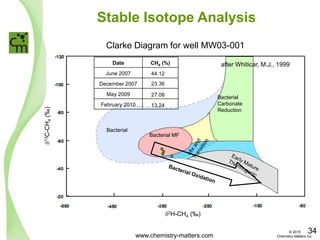
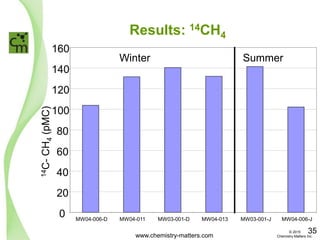
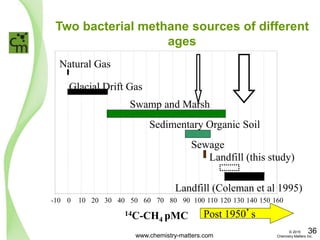
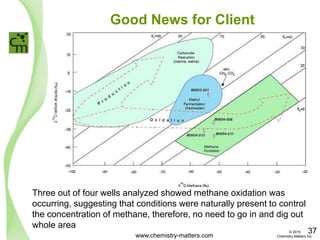
The document discusses a forensic investigation of methane emissions in urban Calgary, highlighting the identification of various sources including landfills and sewage. It details the methodology involving sampling and analysis of gas compositions and isotopes to assess the risks associated with methane exposure. The findings indicate that stable and radio isotope analysis successfully identified sources and informed decisions regarding remediation actions.