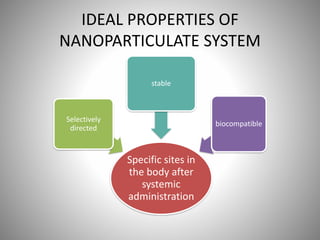
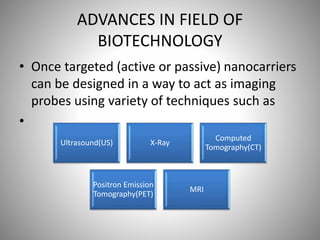
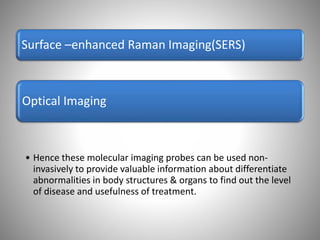
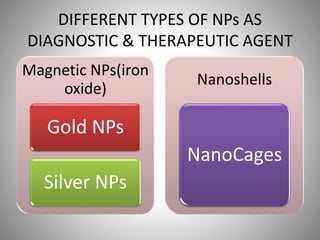
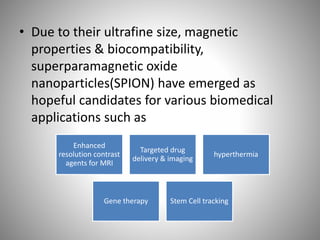
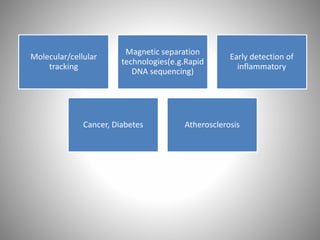
The document discusses the innovations and applications of metallic nanoparticles in drug delivery and imaging for cancer therapy, emphasizing their potential for targeted delivery and enhanced therapeutic index. It outlines the advantages of different types of nanoparticles, including magnetic, gold, and silver nanoparticles, and highlights their ability to overcome challenges faced by traditional chemotherapy. Furthermore, the document explores the concept of theranostics, which combines therapeutic and diagnostic functions, illustrating the transformative role of metallic nanotechnology in oncology.