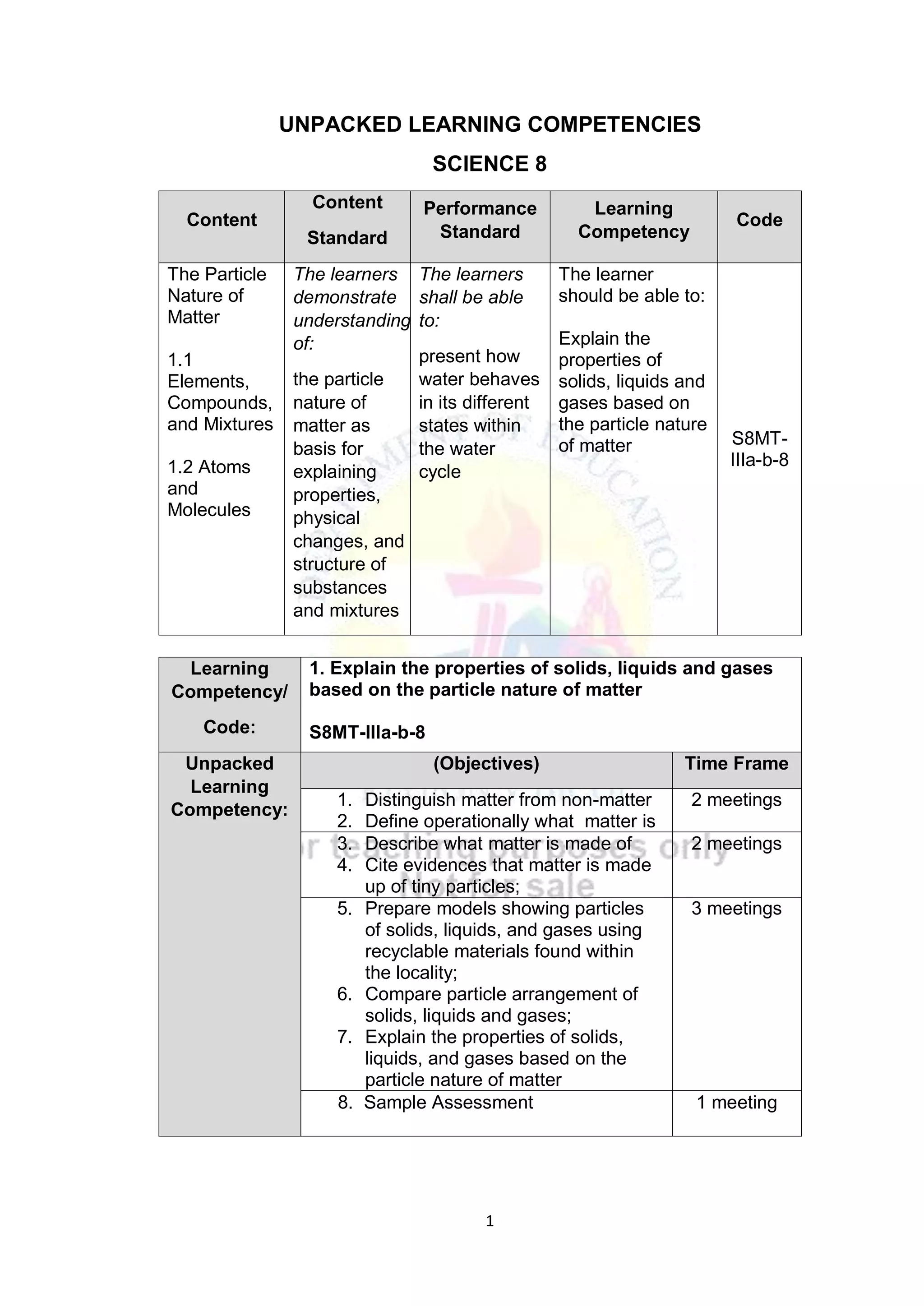
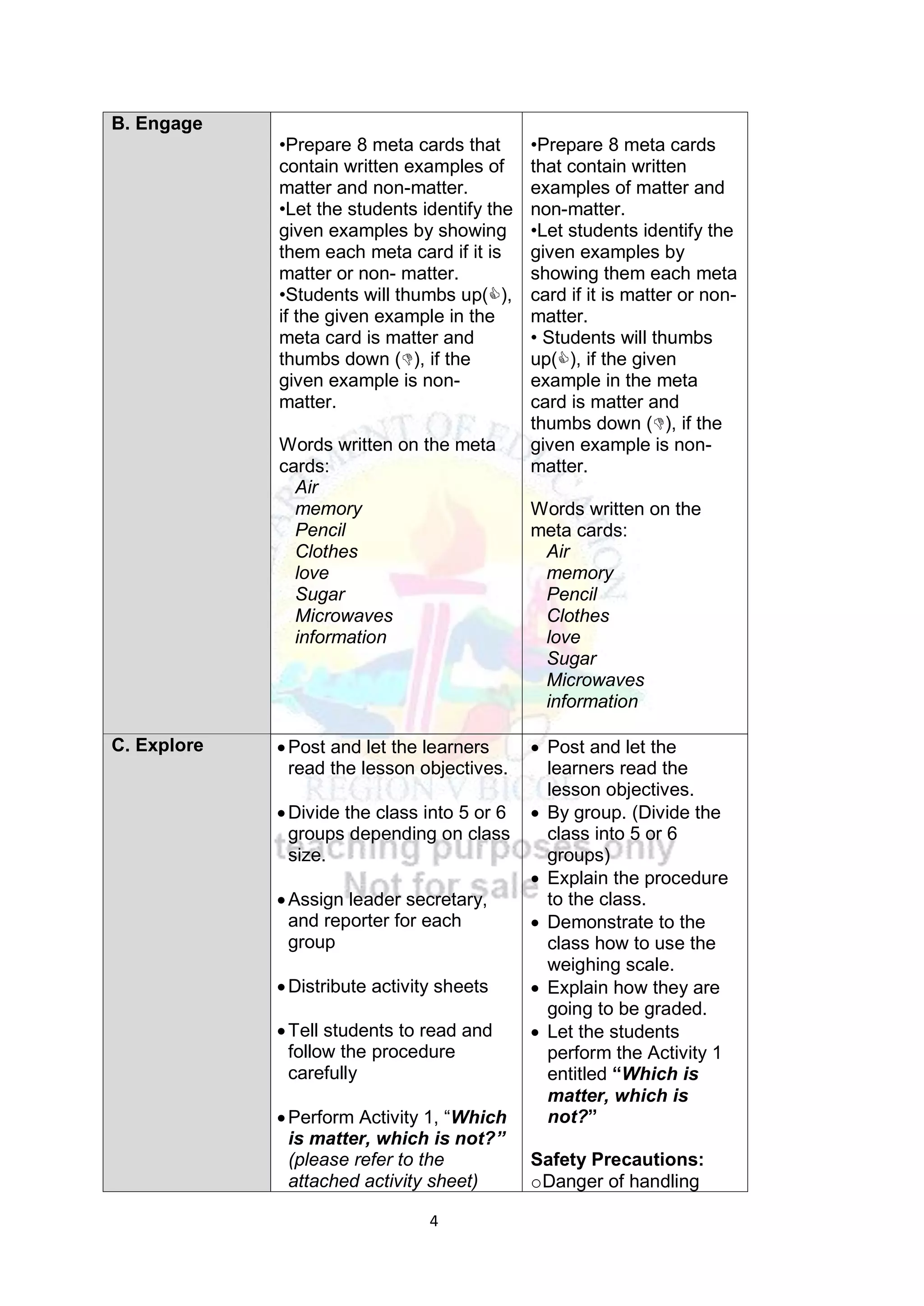
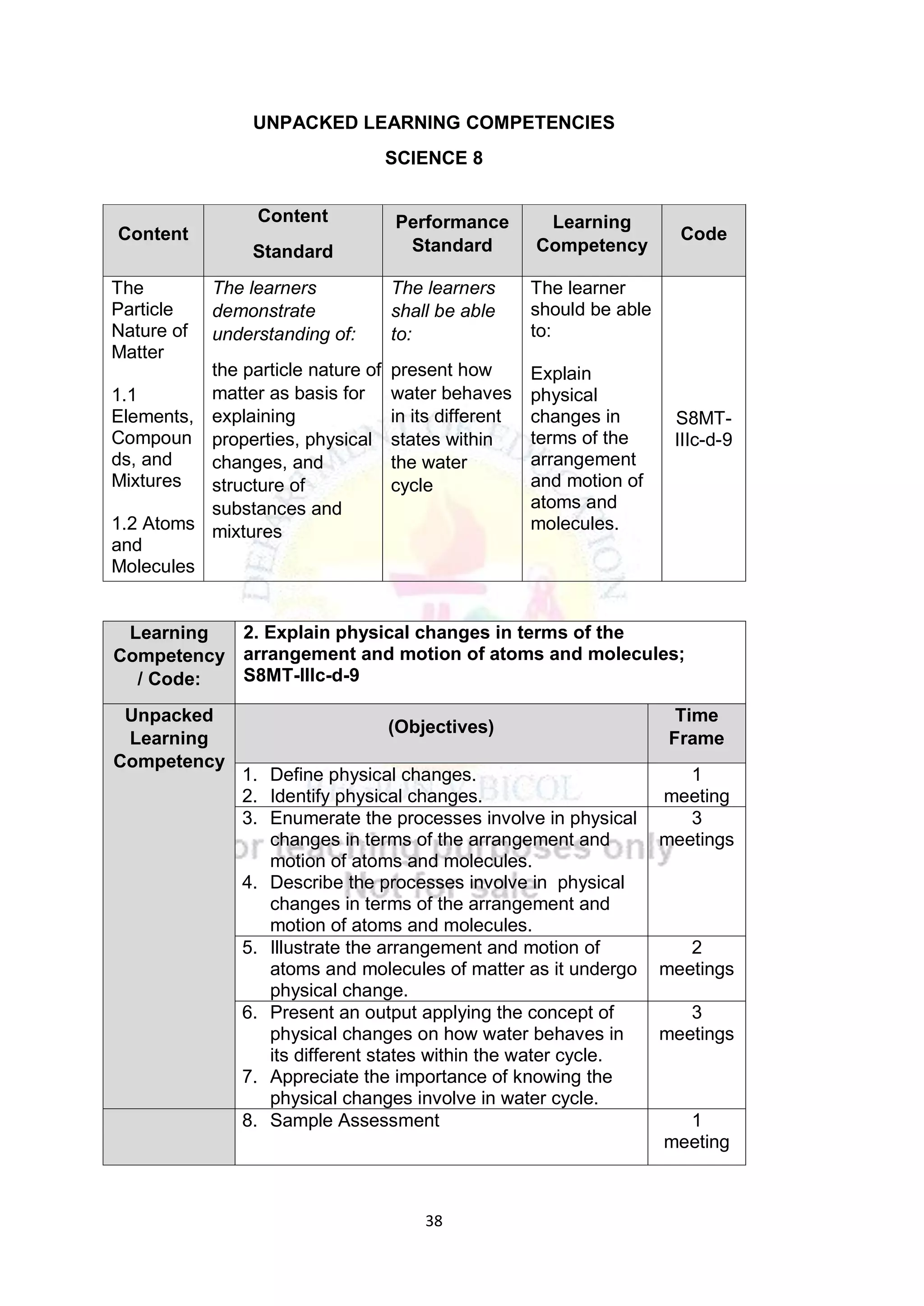
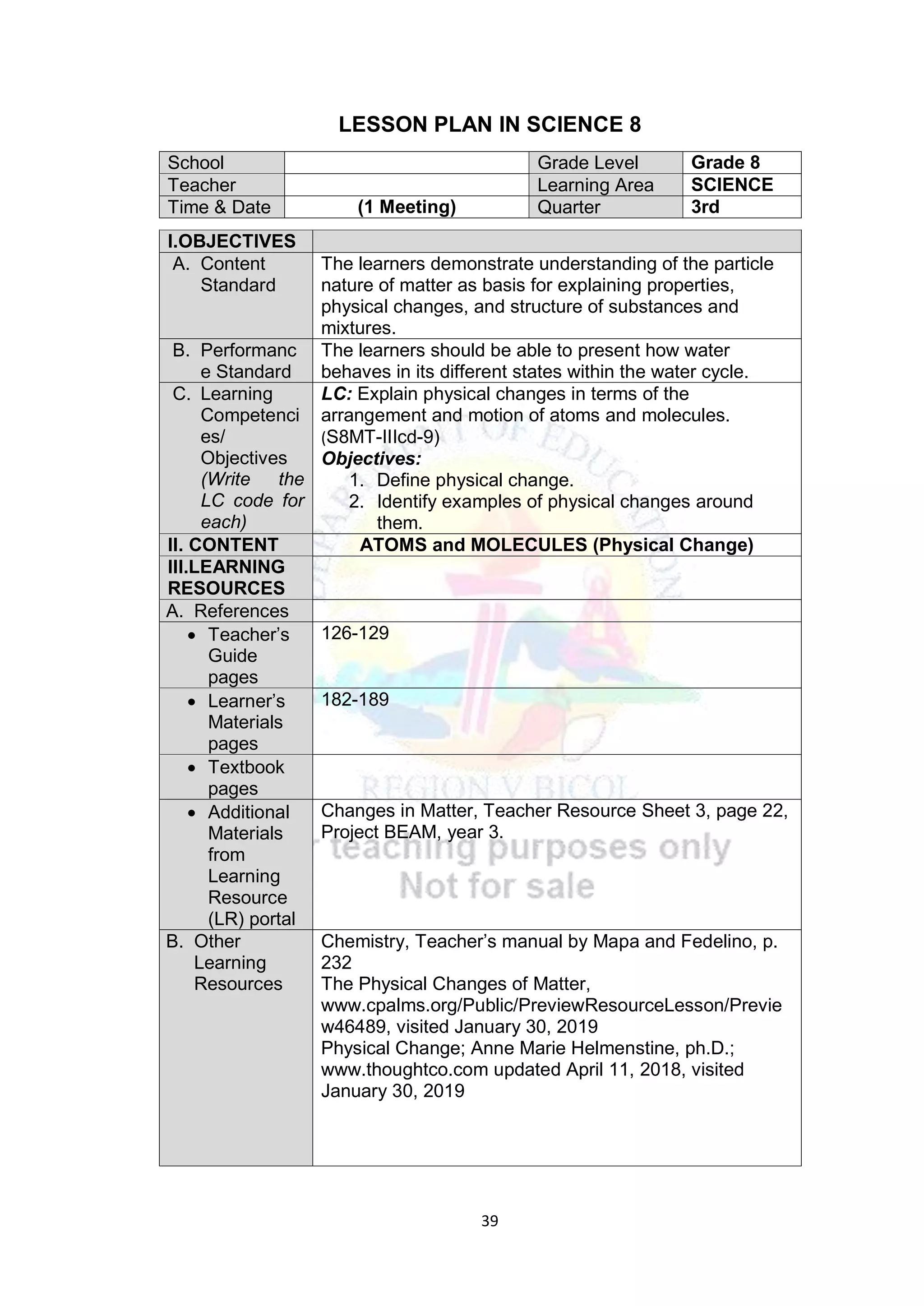
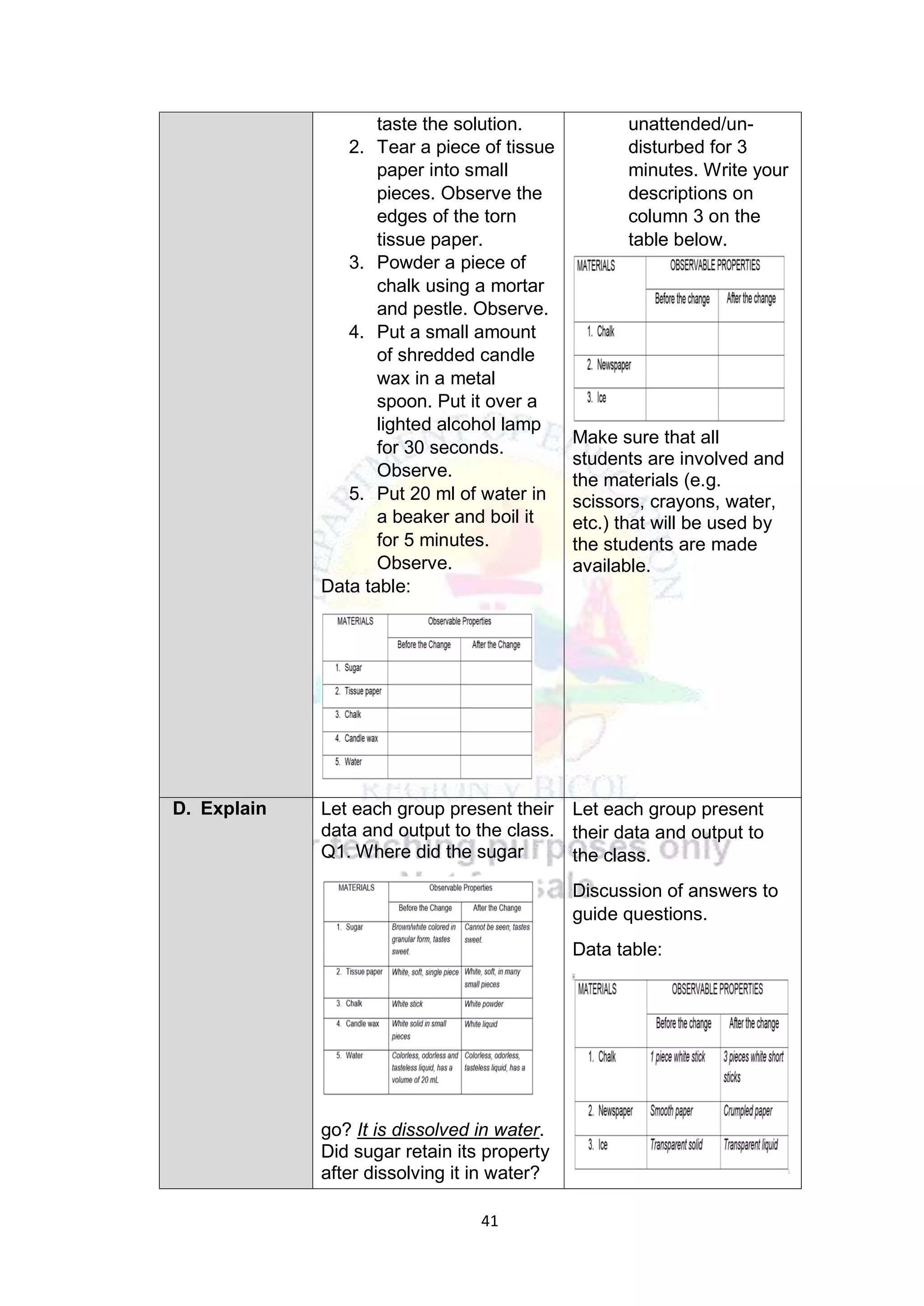
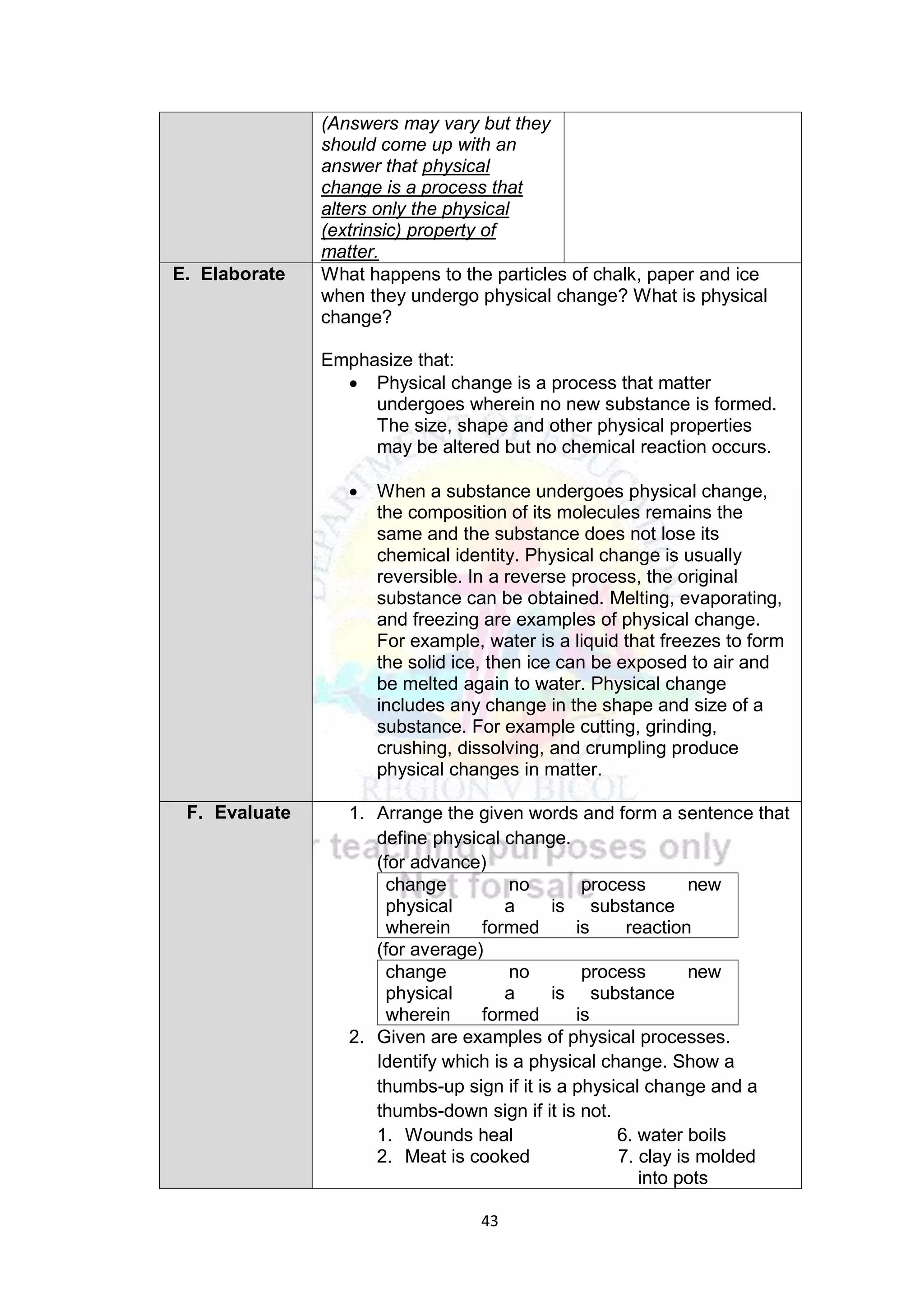
This document contains a lesson plan for teaching 8th grade science students about the particle nature of matter. The lesson plan aims to help students distinguish between matter and non-matter, define what matter is, and explain the properties of solids, liquids, and gases based on the particle nature of matter. The lesson involves students participating in hands-on activities to classify examples as matter or non-matter and identify particle arrangements in the different states of matter using models. It assesses students ability to correctly classify additional examples of matter and non-matter and explain the key differences between the two.





















































































































































































































































![252
IV. PROCEDURE A B
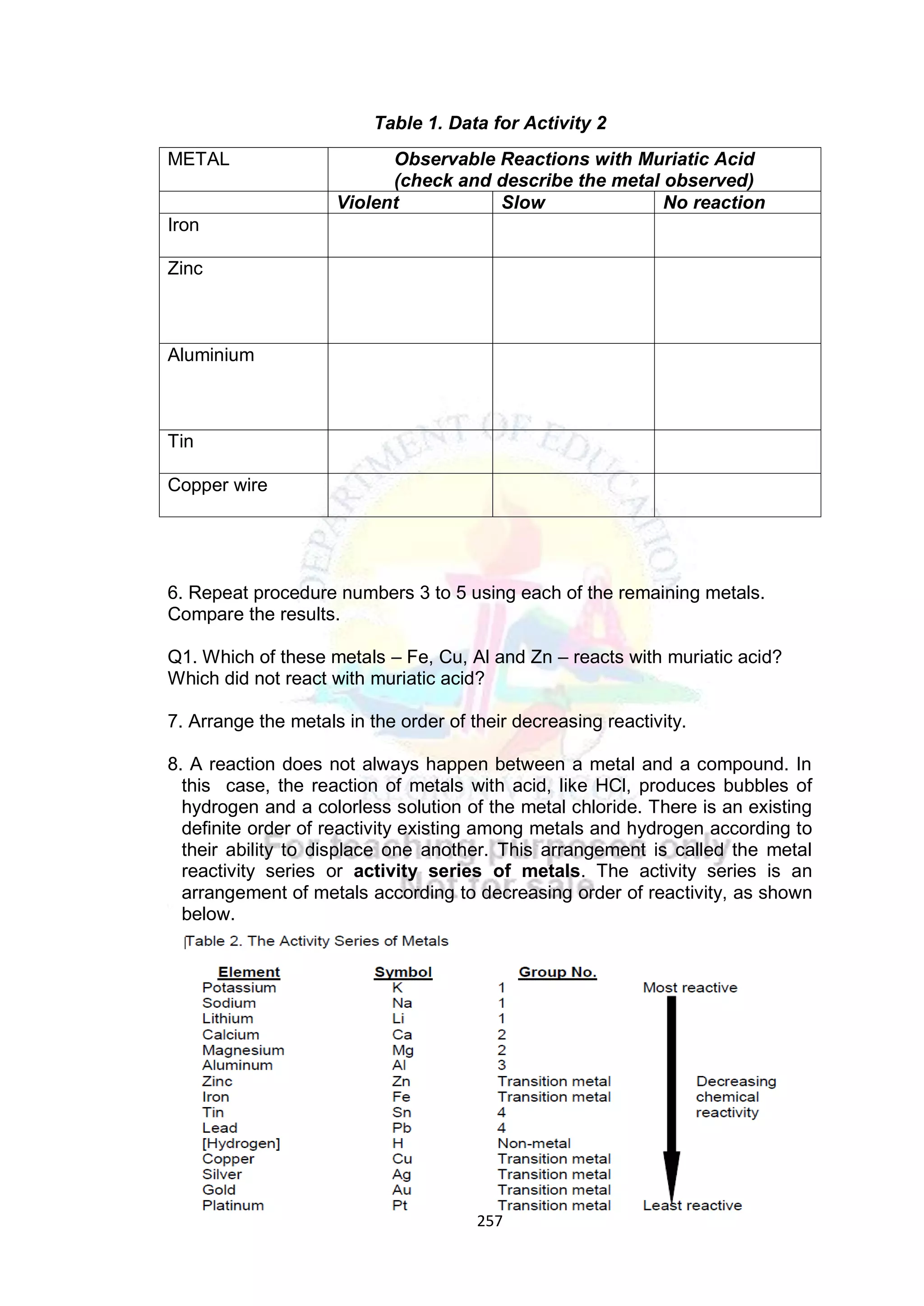
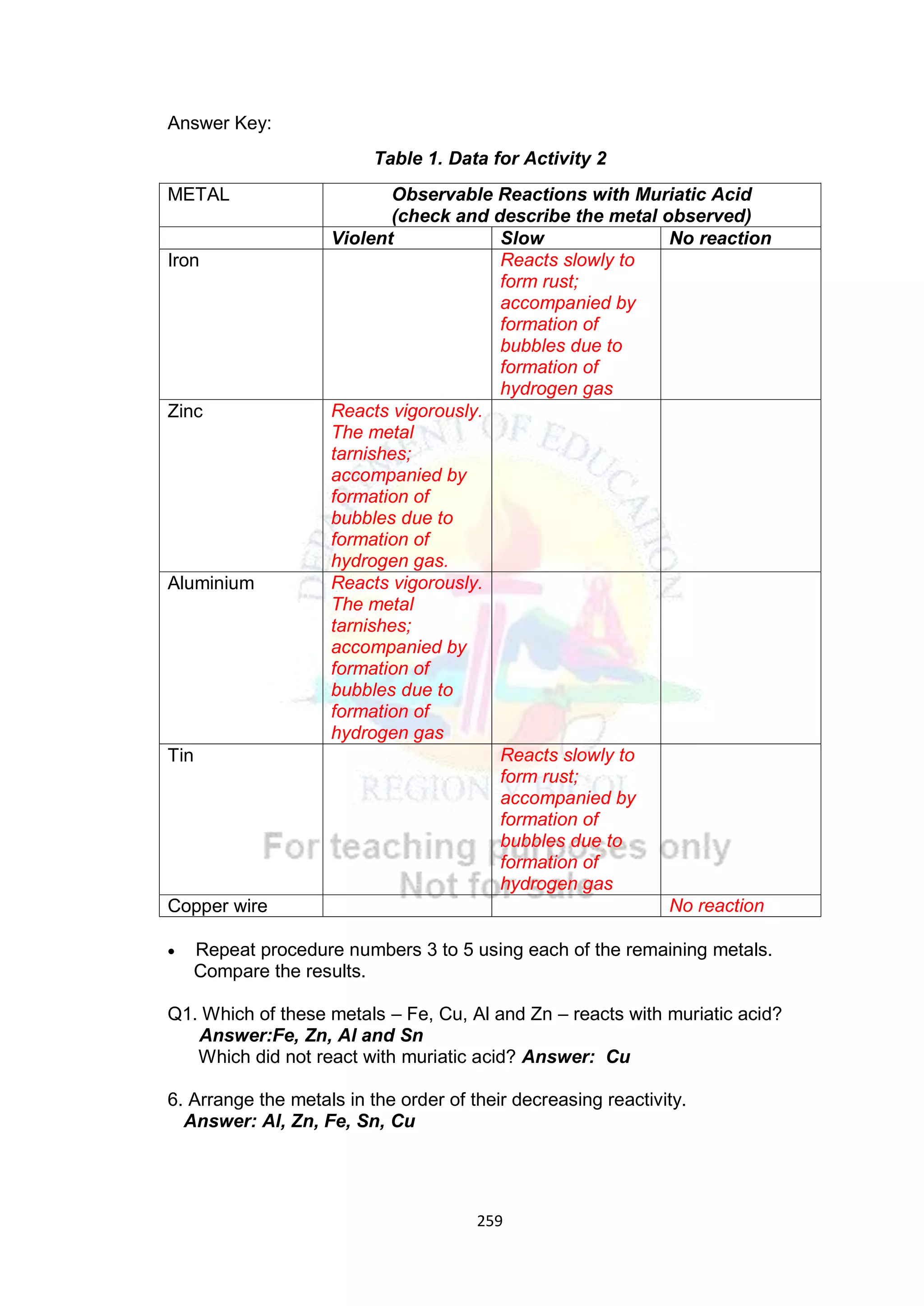
A. Elicit (day 1) Arrange the elements
based on increasing
metallic character:
a. Cs, Fr, Cu
b. Na, Ag, Al
c. Al, Ag, Au
d. Cs, Au, Fe
Answer key
a. Cu, Cs, Fr
b. Al, Ag, Na
c. Al, Au, Ag
d. Au, Fe, Cs
What happens to the
metallic property of the
elements across the
period and from top to
bottom of the periodic
table.
B. Engage Practice Drill: (Recall)
A. Fact or A Bluff!
1. Calcium is more
metallic than Lithium.
[Bluff (Li)]
2. Oxygen is more
nonmetallic than
Fluorine [Bluff (F)]
3. Sodium is more
metallic than
Magnesium? [Fact(Na)]
4. Gold is more metallic
than Silver. [Bluff(Ag)]
5. Sodium is more
reactive than
Potassium. [Bluff (K)]
Practice Drill: (Recall)
A Fact or A Bluff!
1. Calcium is more
metallic than Lithium.
[Bluff (Li)]
2. Oxygen is more
nonmetallic than
Fluorine [Bluff (F)]
3. Sodium is more
metallic than
Magnesium?
[Fact(Na)]
4. Gold is more metallic
than Silver. [Bluff(Ag)]
5. Sodium is more
reactive than
Potassium. [Bluff (K)]
C. Explore The class will be divided
into five groups.
Refer to LM activity
number 2 pages 214-215.
Activity Title: Metal…Metal
How reactive are you?
Students will perform the
said activity.
Note: Each group will
provide their own
materials listed in the
book.
The class will be divided
into five groups.
Refer to LM activity
number 2 pages 214-215.
Activity Title: Metal…Metal
How reactive are you?
Students will perform the
said activity.
Note: Each group will
provide their own
materials listed in the
book.](https://image.slidesharecdn.com/science8q3-230926135416-82312da9/75/Science-8-Q3-pdf-258-2048.jpg)