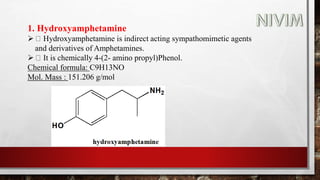
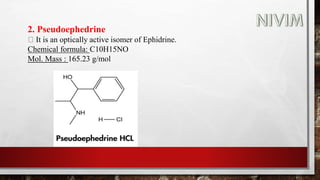
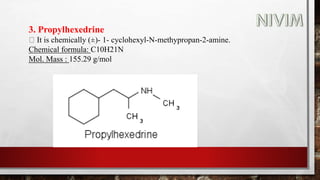
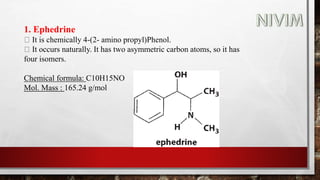
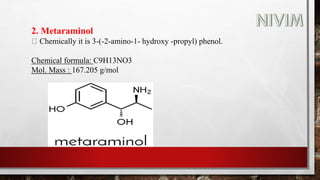
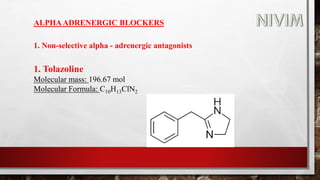
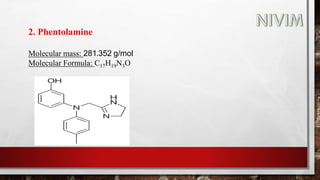
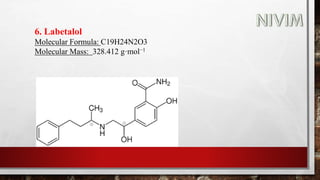
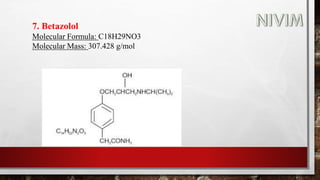
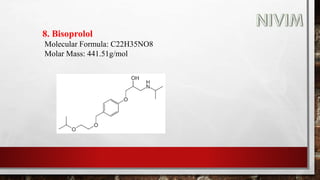
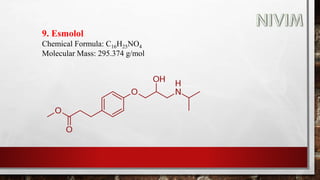
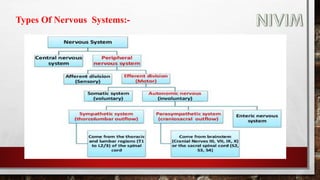
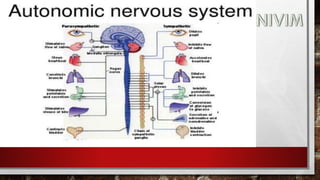
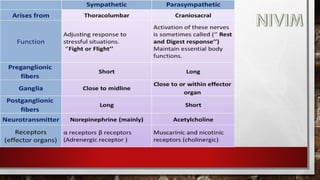
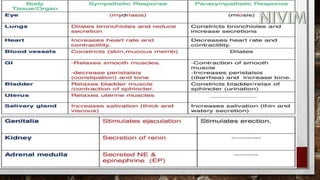
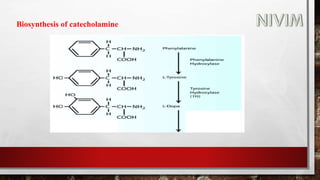
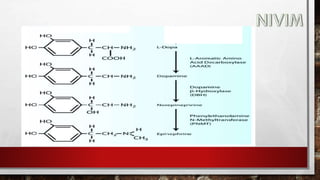
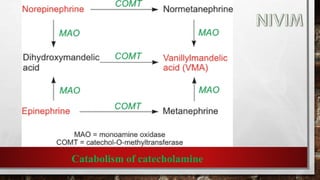
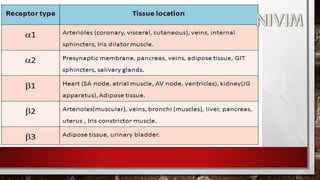
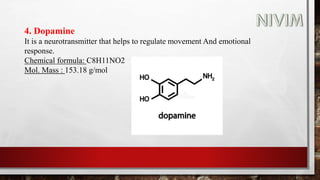
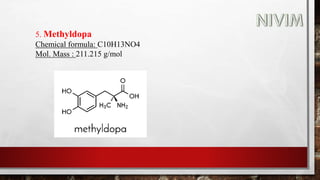
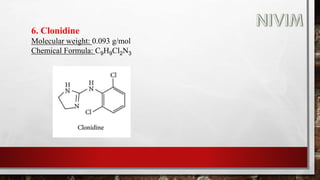
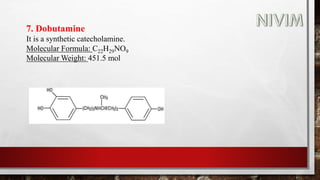
The document provides an overview of the autonomic nervous system, including its structure, function, and neurotransmitters involved, such as norepinephrine and acetylcholine. It also covers adrenergic neurotransmitters, their biosynthesis, catabolism, and receptor distribution, highlighting sympathomimetic agents and their mechanisms. The structure-activity relationships of these compounds are discussed, detailing their pharmacological actions and clinical applications.




































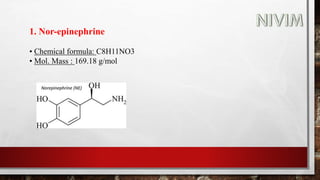



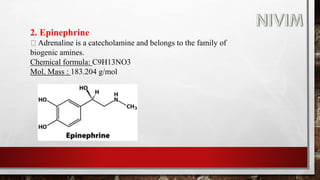




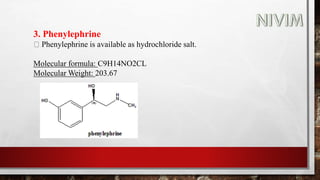

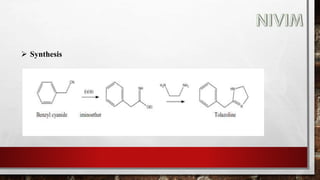
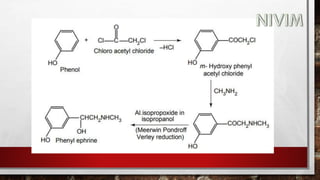
![Synthesis:
Phenol with 2-chloro acetyl chloride gives 2-chloro -1[3-hydroxy
phenyl]ethanone.(M-Chloroacetylphenol)
2-chloro -1[3-hydroxy phenyl] ethanone react with NH2CH3 it gives
1-(3- hydroxyphenyl)2- (methyl amino) ethanone.
1-(3-hydroxyphenyl)2- (methyl amino) ethanone under go reduction
reactions (addition of hydrogen) to form Phenylephrine.](https://image.slidesharecdn.com/unit-2-221129034224-217c9aec/85/Medicinal-Chemistry-Unit-2-pptx-56-320.jpg)









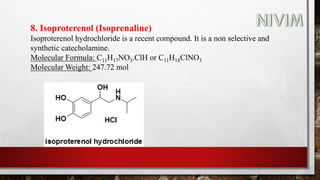


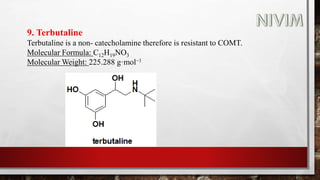


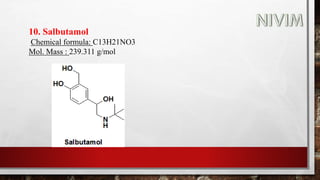



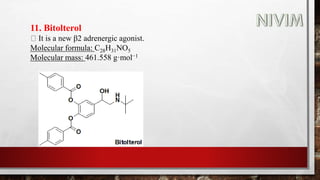


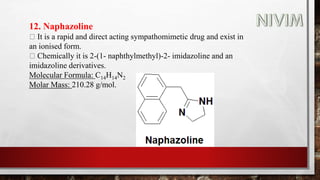



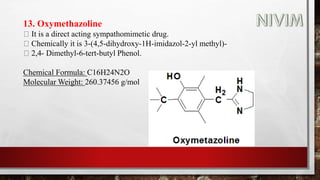

![14. Xylomethazoline
It is a direct acting sympathomimetic drug.
Chemically it is 2-[(4-tert-butyl-2,6-dimethyl phenyl)methyl]-4,5
dihydro-lH-imidazole.
Molecular Formula: C16H24N2
Molecular Weight: 244.37 mol](https://image.slidesharecdn.com/unit-2-221129034224-217c9aec/85/Medicinal-Chemistry-Unit-2-pptx-90-320.jpg)