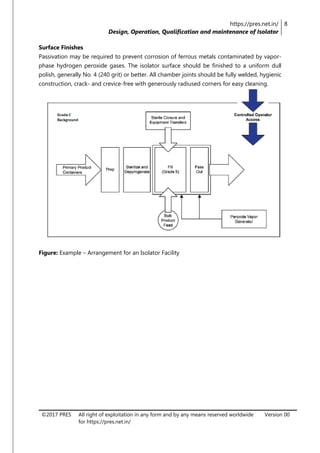
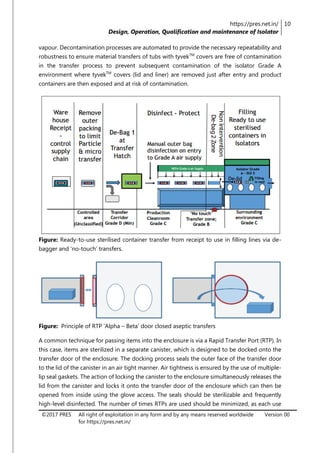
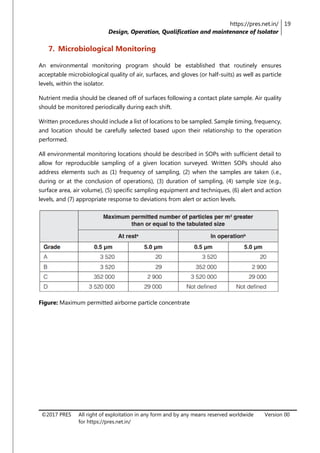
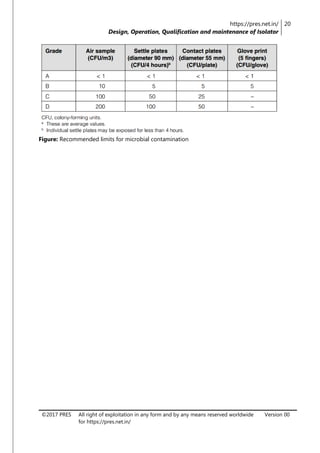
The document is a review article focusing on the design, operation, qualification, and maintenance of aseptic processing isolators in the pharmaceutical industry. It highlights the significance of isolators in creating a controlled environment for sterile product handling and details various aspects such as applications, cleaning, decontamination, and monitoring of isolators. The article also emphasizes the importance of proper design and operator training to minimize contamination risks associated with aseptic processing.