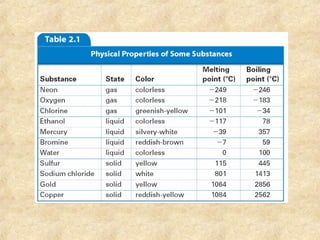
1. The document discusses various properties of matter and how they are used to classify and identify different types of matter. It describes extensive properties that depend on amount and intensive properties that depend on type.
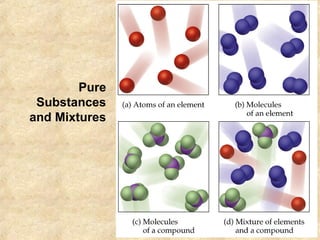
2. Mixtures and pure substances are introduced. Heterogeneous mixtures are non-uniform while homogeneous mixtures are uniform throughout. Elements have a unique set of properties while compounds contain two or more elements.
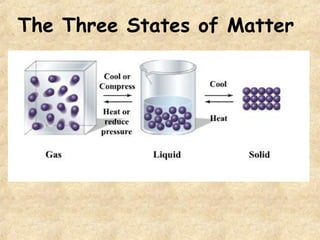
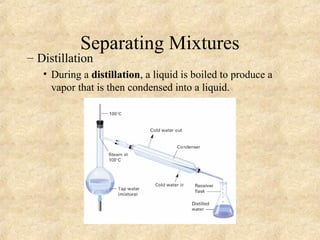
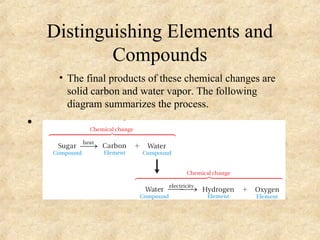
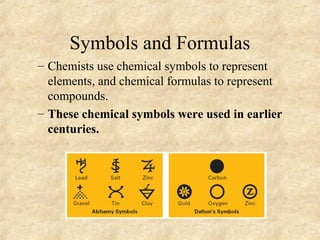
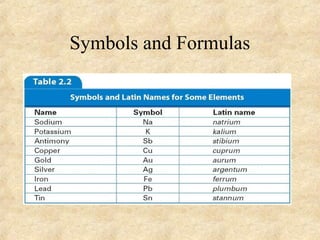
3. The three states of matter are defined as solid, liquid, and gas. Physical and chemical changes are distinguished based on whether the composition changes. Chemical symbols and formulas are used to represent elements and compounds in chemical reactions.