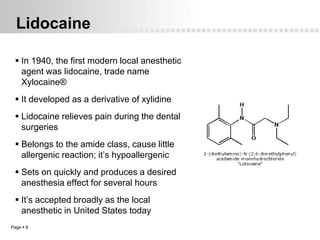
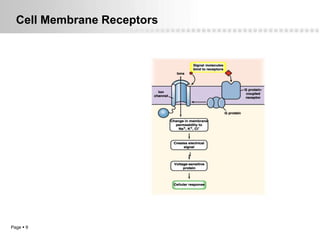
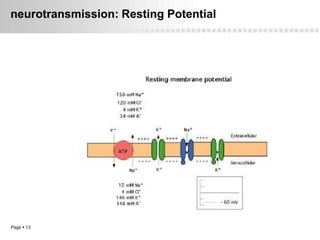
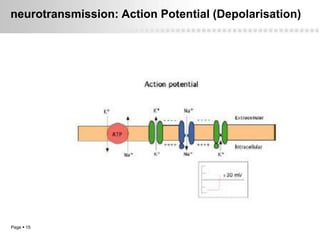
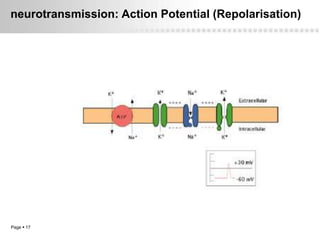
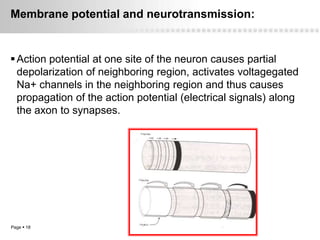
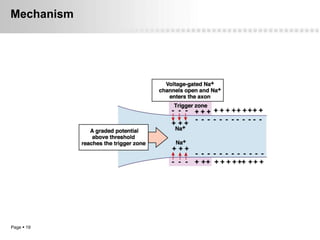
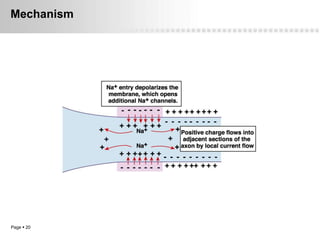
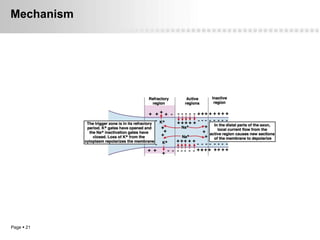
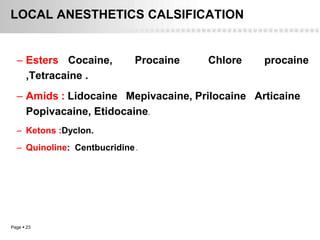
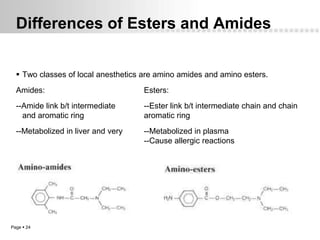
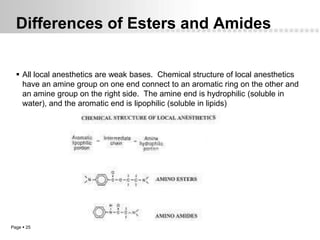
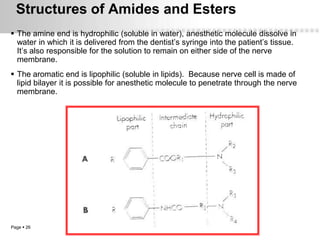
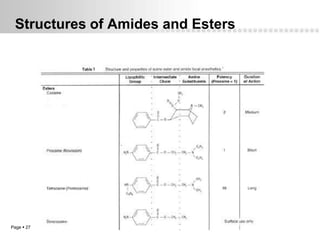
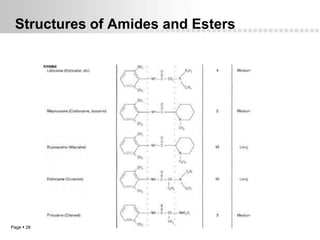
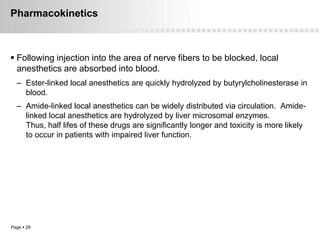
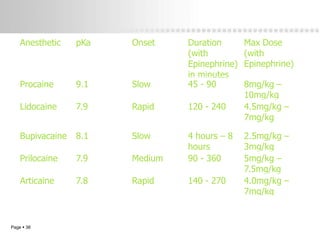
Local anesthetics work by altering the membrane potential of nerve cells and blocking sodium channels, which prevents the propagation of action potentials and results in loss of sensation. Early local anesthetics like cocaine were derived from coca leaves and provided pain relief for surgery. Later developments included procaine, lidocaine, and other synthetic amide and ester local anesthetics. The pharmacokinetics and effects of local anesthetics depend on factors like lipid solubility, pH, vasoconstriction, and metabolism. Toxicity can occur if maximum dosage levels are exceeded.