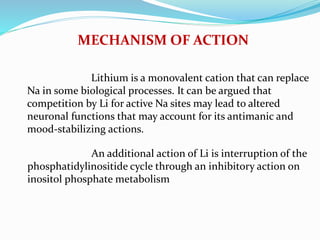
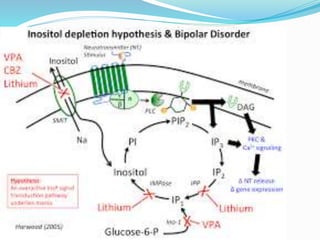
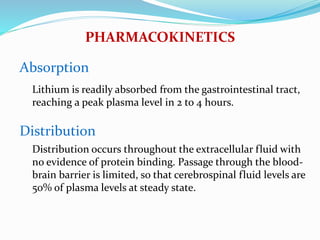
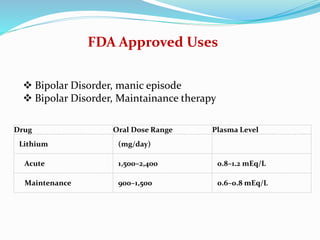
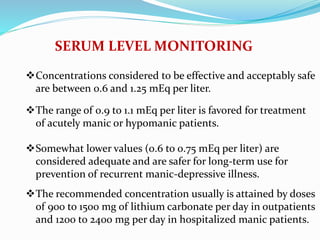
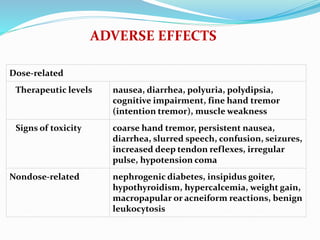
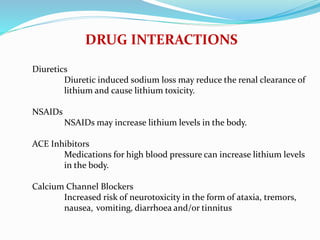
Lithium was first introduced as a treatment for mania in 1949 and approved by the FDA for this purpose in 1970. It is effective for treating manic episodes and preventing recurrent manic/depressive episodes in bipolar patients. Lithium is readily absorbed and distributed throughout extracellular fluid, reaching peak plasma levels within 2-4 hours. It is excreted primarily through the kidneys, with an elimination half-life of around 24 hours. Therapeutic lithium levels range from 0.6-1.2 mEq/L, depending on whether it is being used to treat an acute episode or for long-term maintenance. Adverse effects are generally dose-related and include nausea, tremors, and