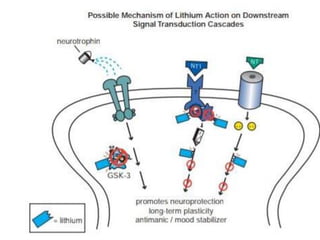
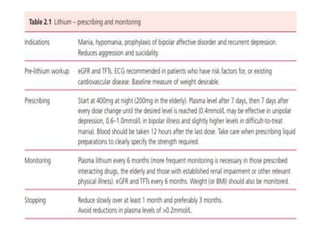
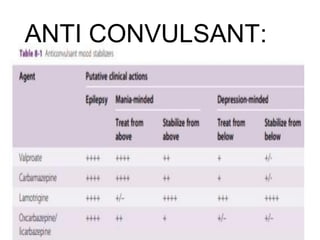
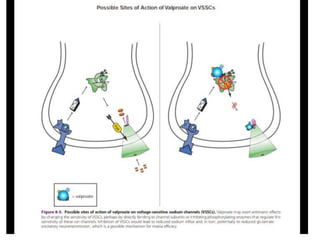
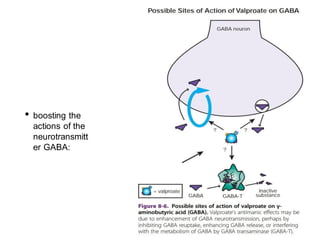
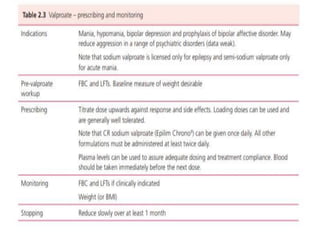
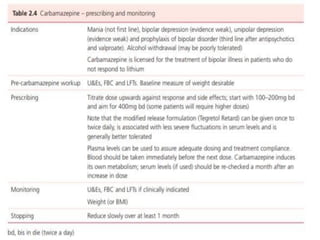
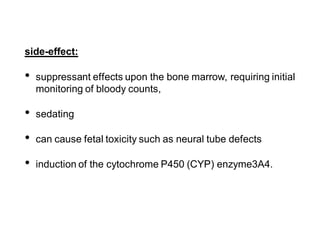
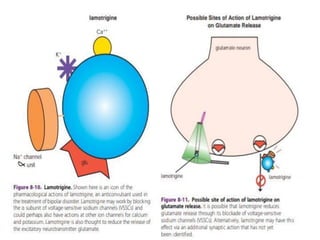
This document discusses mood stabilizers including lithium, various anticonvulsants, and atypical antipsychotics. It provides in-depth information on the mechanisms of action, clinical indications, dosing, monitoring, side effects, and toxicity of lithium and sodium valproate. Lithium is effective for manic episodes, suicide prevention, and maintenance treatment in bipolar disorder. Valproate is proven effective for acute mania and commonly used long-term to prevent manic recurrence. Both require monitoring of plasma levels and side effects like gastrointestinal issues, tremors, and potential renal and thyroid impacts.