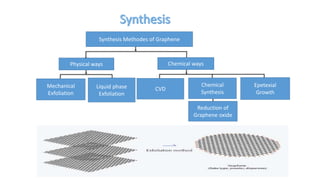
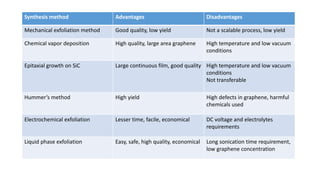
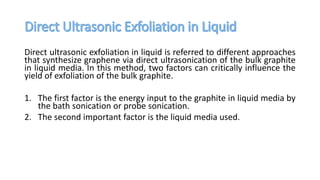
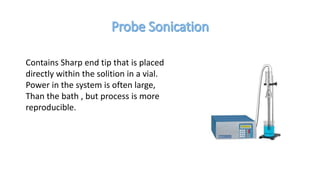
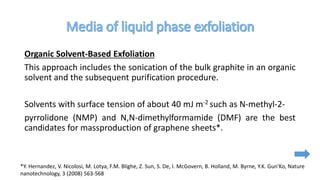
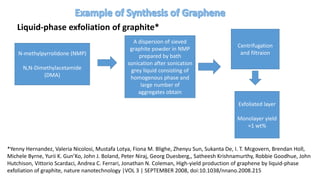
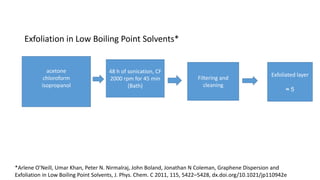
The document discusses various methods for synthesizing graphene, with a focus on liquid phase exfoliation. It describes how liquid phase exfoliation involves ultrasonically exfoliating graphite in liquid media such as organic solvents or ionic liquids. Direct ultrasonication can influence the exfoliation yield, depending on energy input and the liquid media used. Sonication applies sound energy to agitate particles and is usually done with an ultrasonic bath or probe. Common solvents for liquid phase exfoliation include NMP and DMF. Low boiling point solvents like isopropanol and chloroform have also shown success. Liquid phase exfoliation has advantages such as being economical and scalable compared to other methods