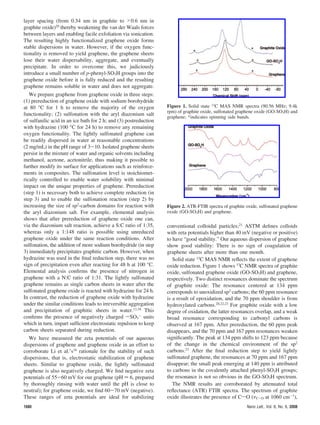
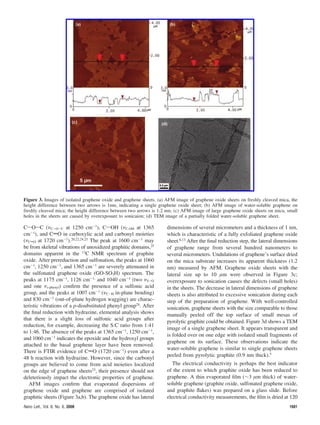
This document summarizes a method for synthesizing water-soluble graphene through a three step process: 1) prereduction of graphene oxide using sodium borohydrate to remove most oxygen functionality, 2) controlled sulfonation using aryl diazonium salt to introduce sulfonic acid groups for solubility while minimally impacting graphene properties, and 3) postreduction using hydrazine to remove remaining oxygen functionality. Characterization with 13C NMR and FTIR indicates restoration of the graphene sp2 carbon network. The introduced sulfonic acid groups allow individually dispersed graphene sheets in water, maintaining stability through electrostatic repulsion.