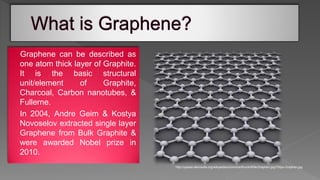
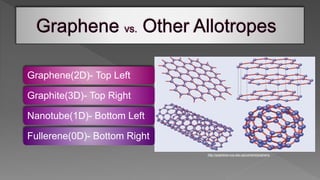
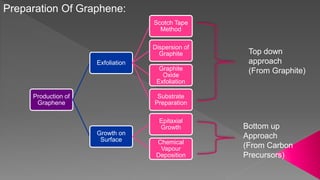
This document is a seminar report submitted by Sunamro Sarkar discussing compounds for pocket-sized data storage. It introduces graphene and its properties, methods for preparing graphene like scotch tape method and chemical vapor deposition. The report discusses how an organometallic compound containing zinc and graphene fragments sandwiched between cobalt and copper layers can store and read digital data at -23°C using molecular spintronics and electron spin orientation. It achieves data storage capacity of 1000 terabytes per square inch using this mechanism.