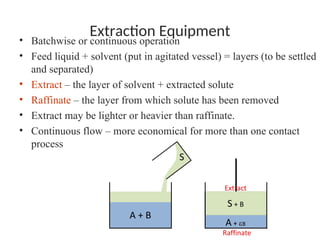
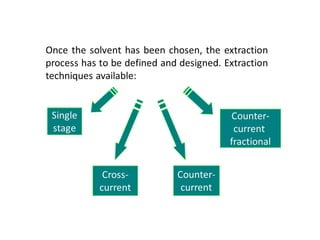
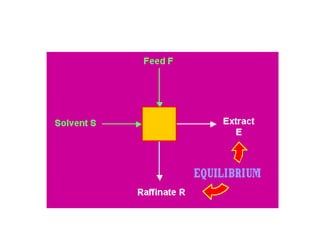
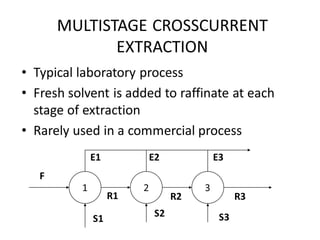
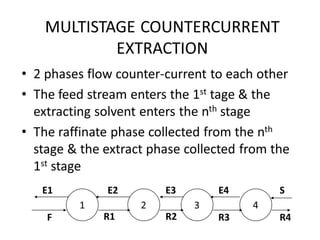
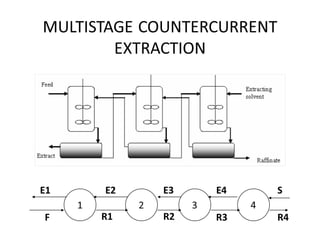
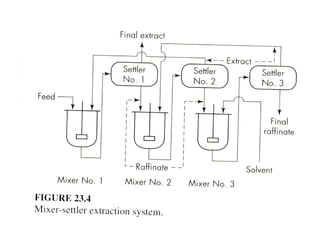
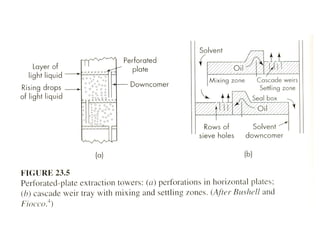
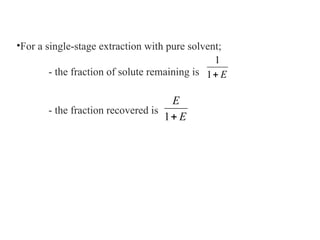
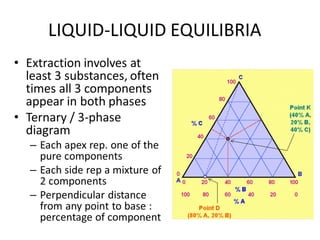
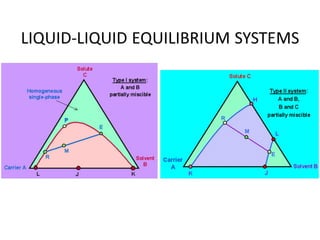
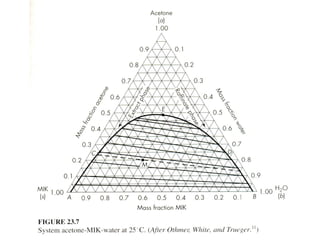
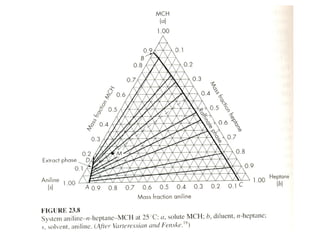
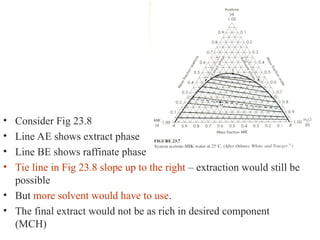
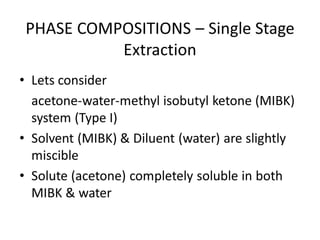
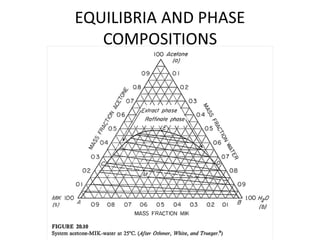
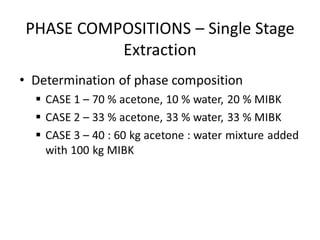
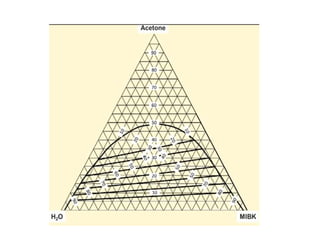
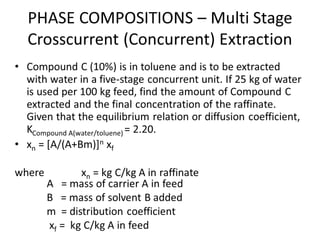
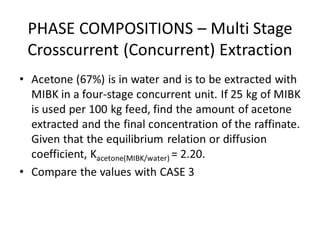
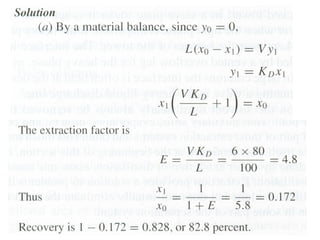
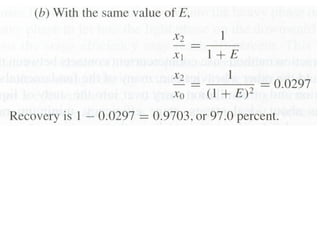The document discusses liquid-liquid extraction, outlining its purpose, equipment, and operational methods. It describes the process of selectively separating components from a mixture using immiscible solvents, and details single-stage and multi-stage extraction processes. Examples, including the extraction of penicillin and acetic acid, are provided, along with mathematical definitions for extraction factors and equilibrium relationships.