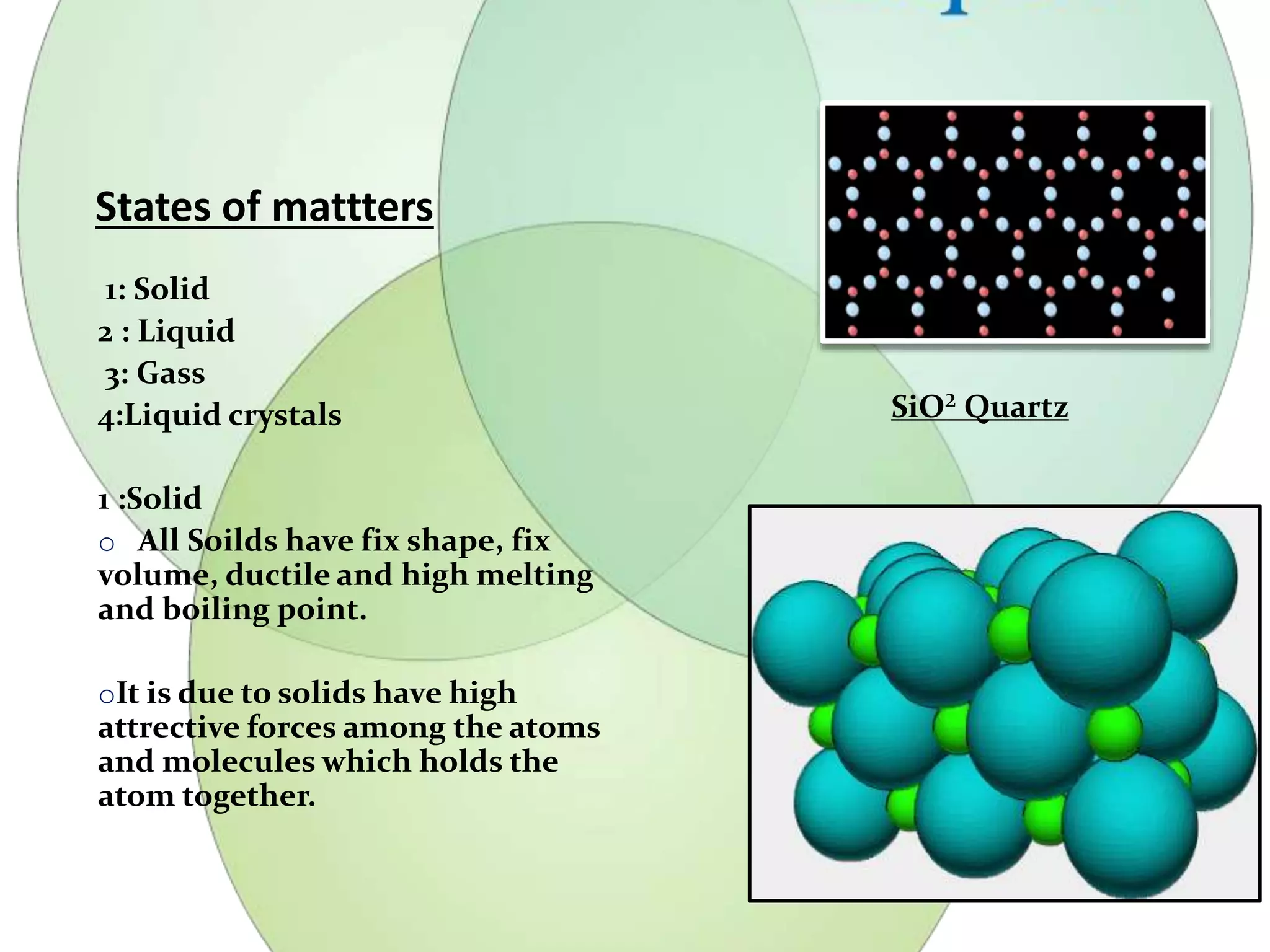
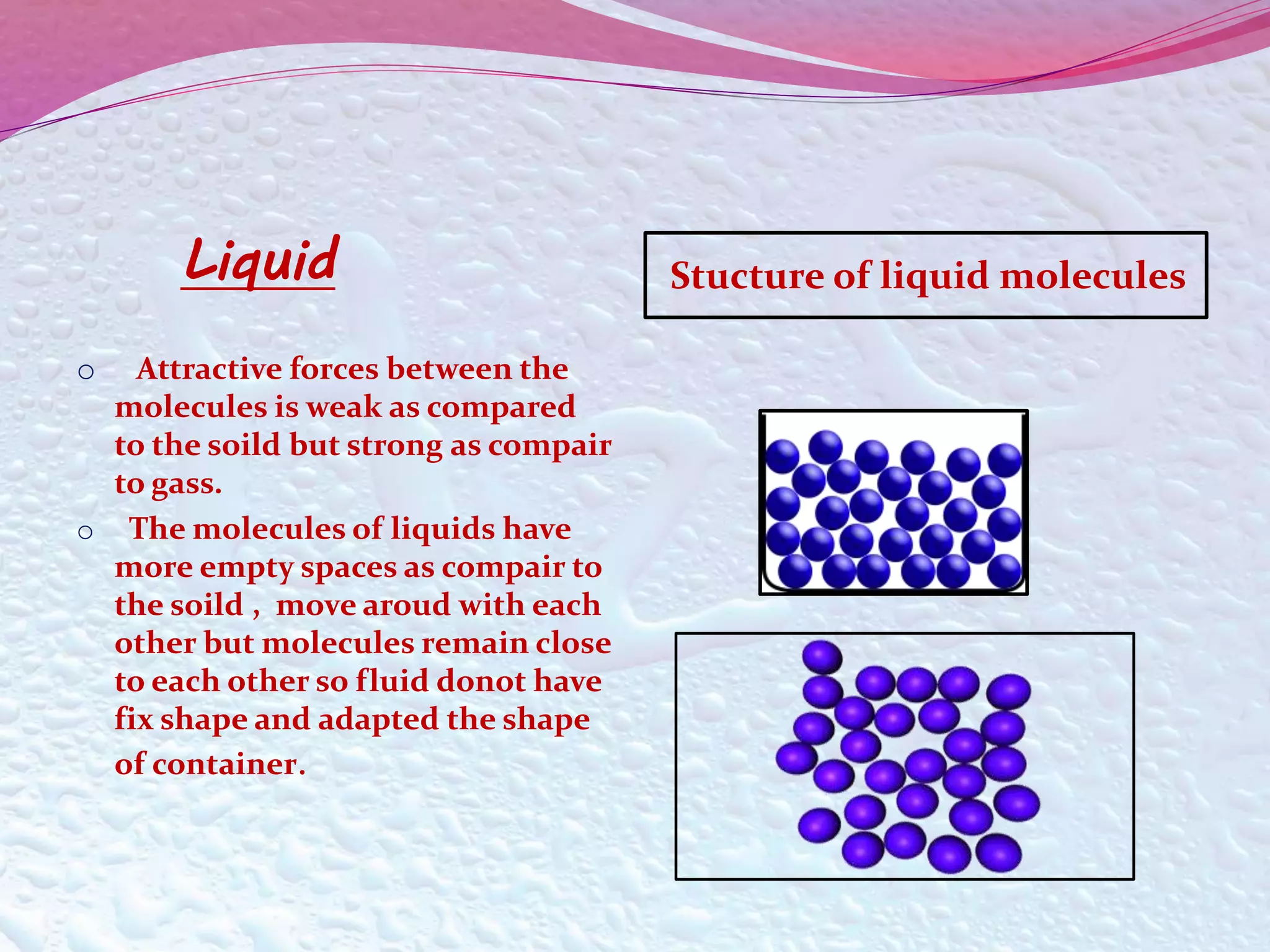
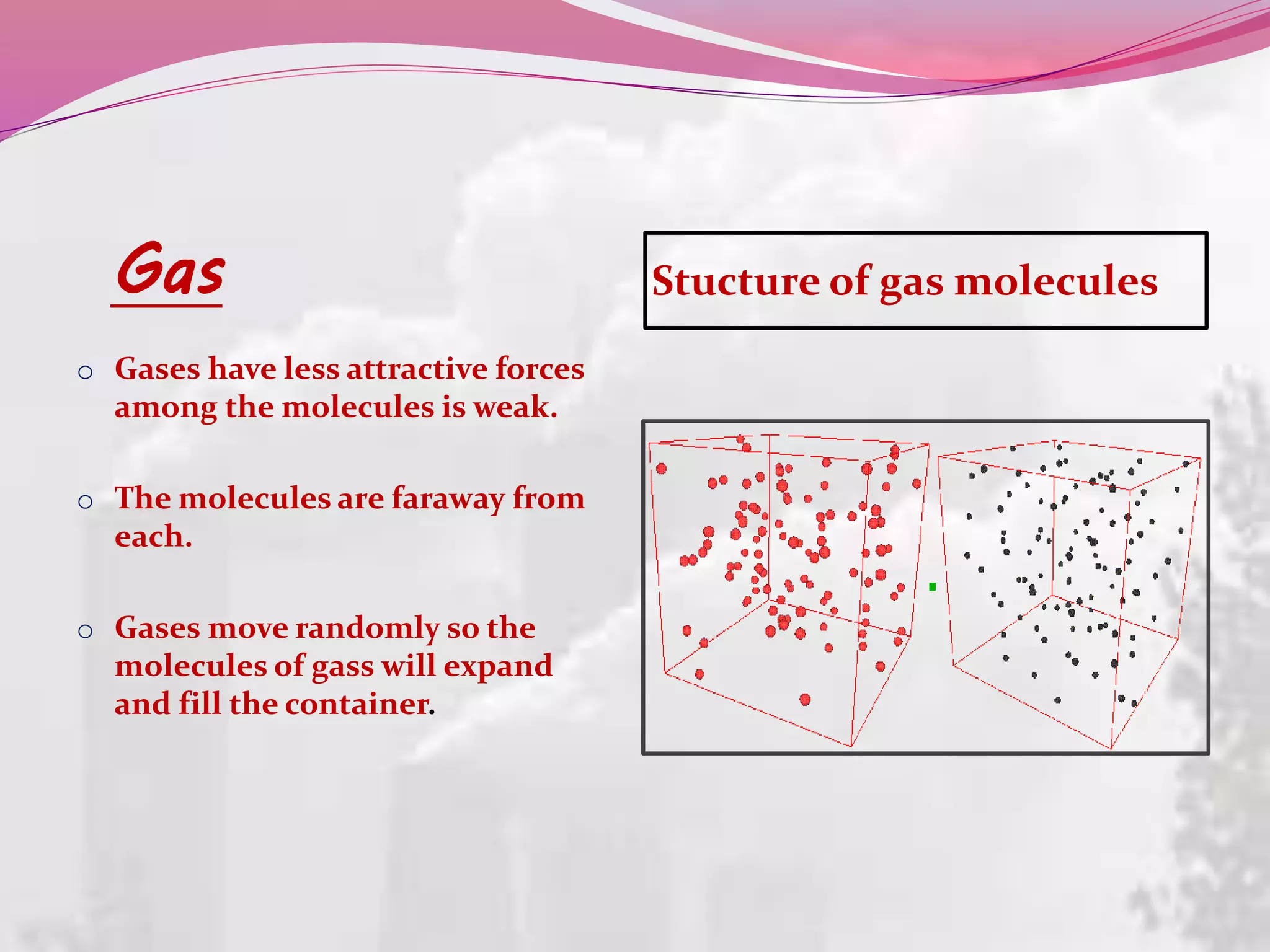
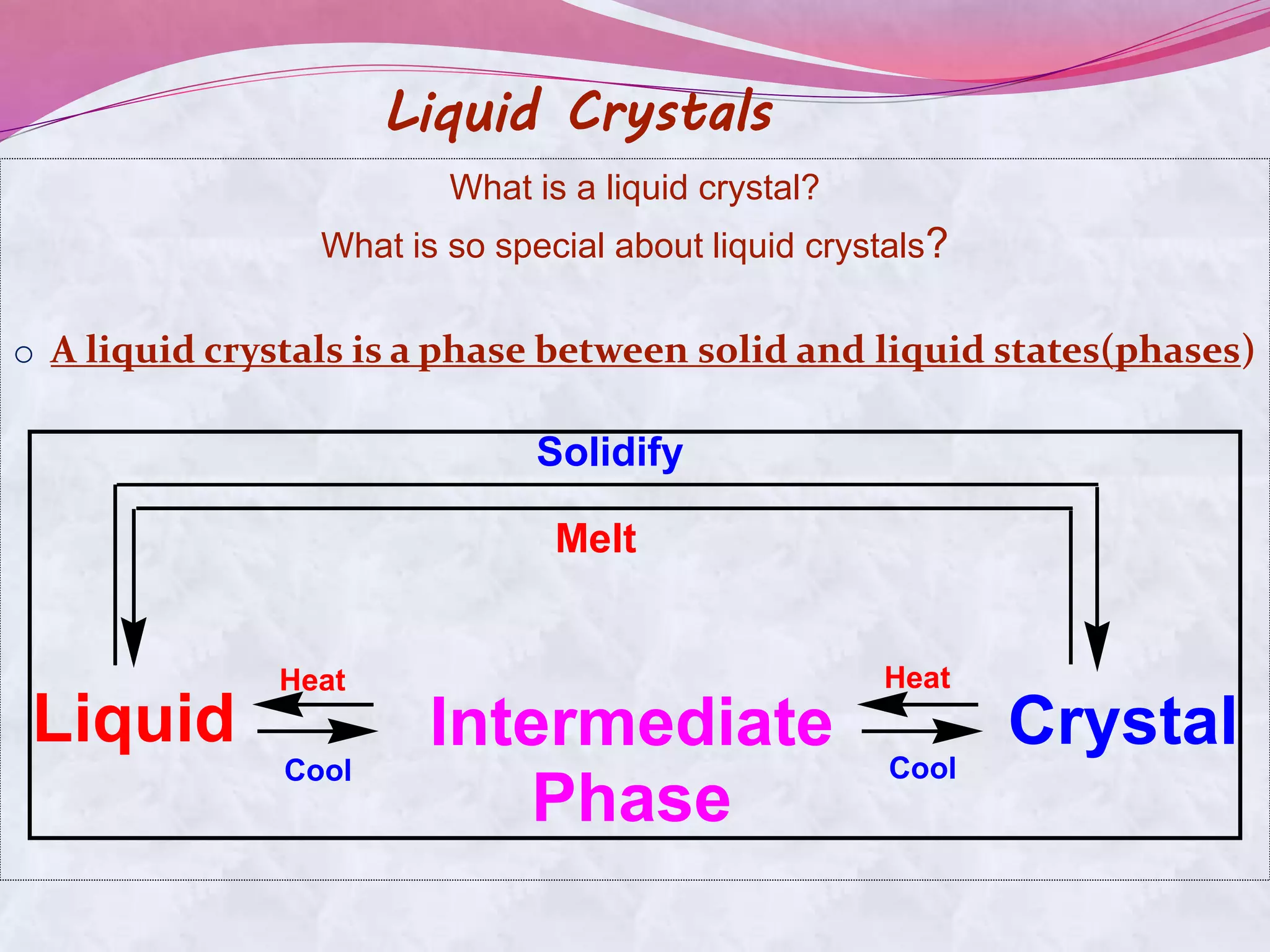
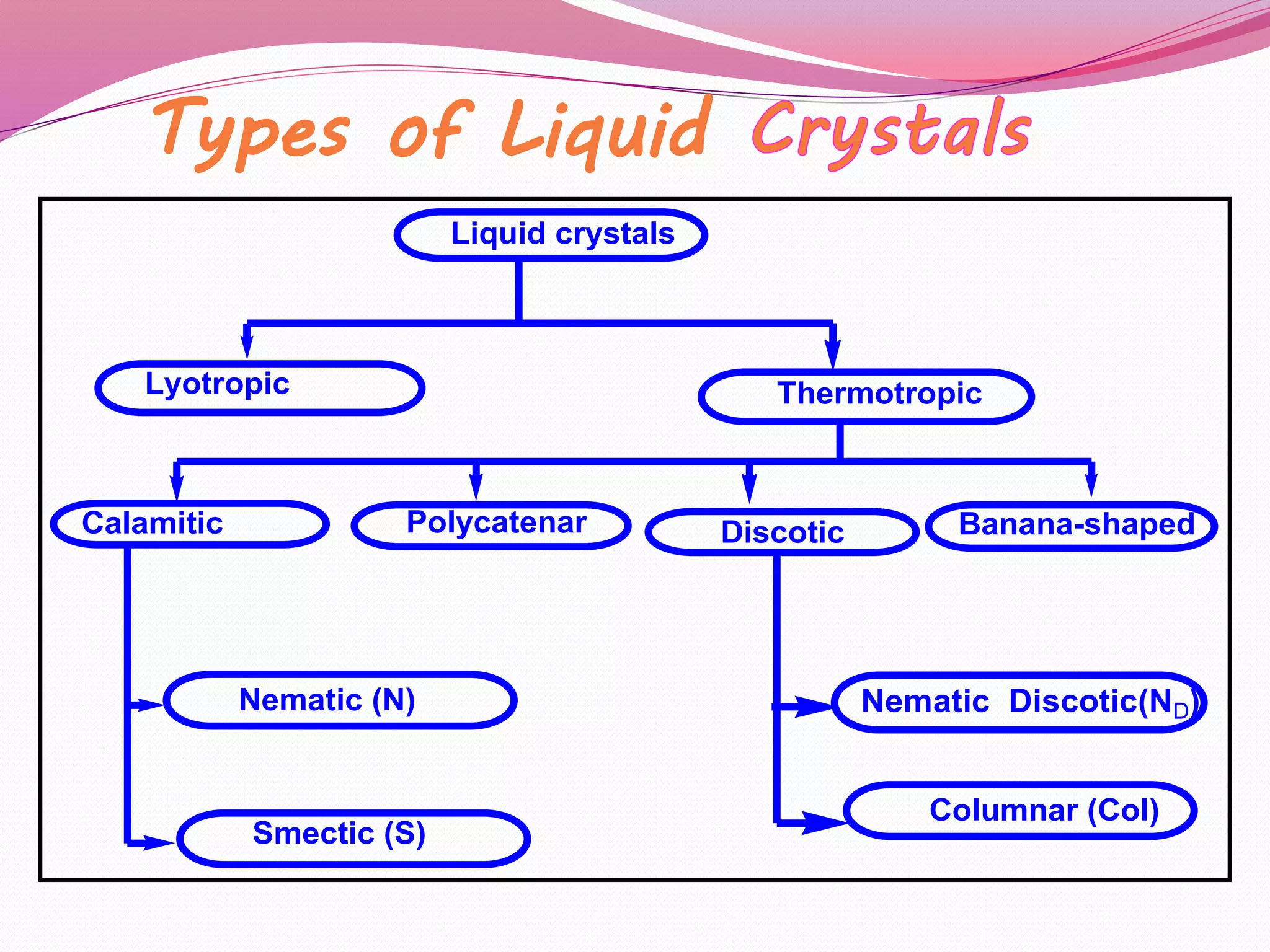
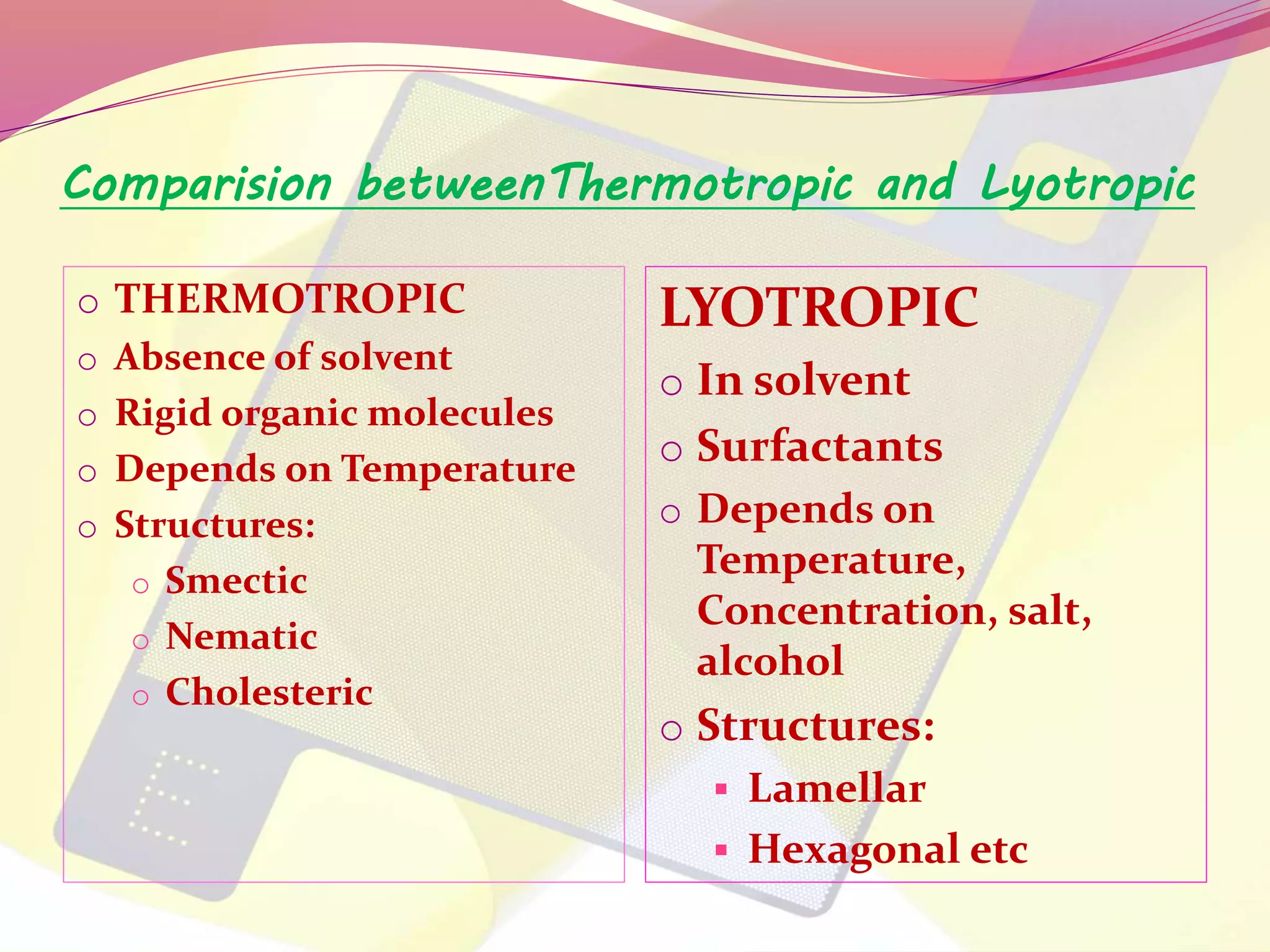
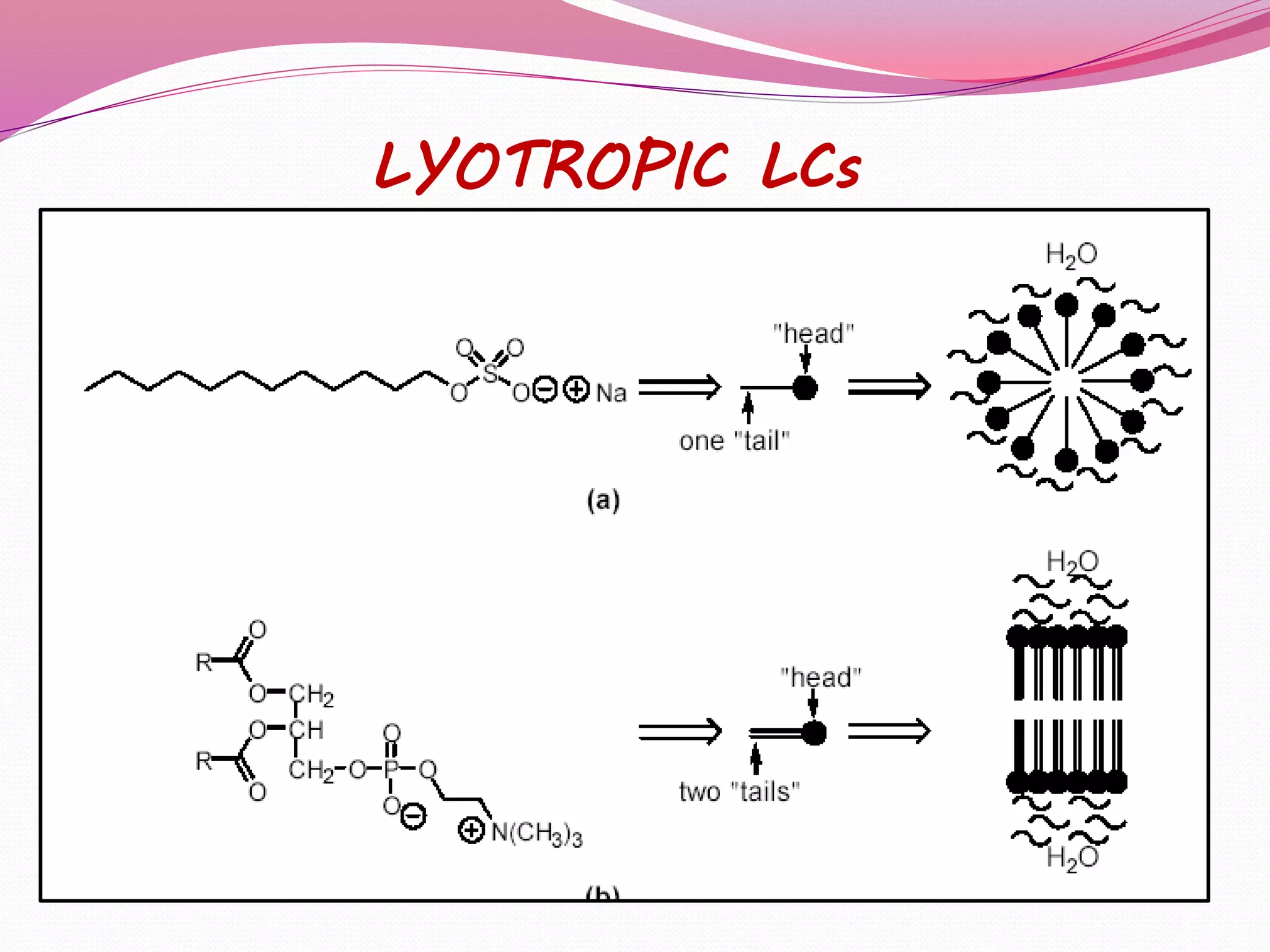
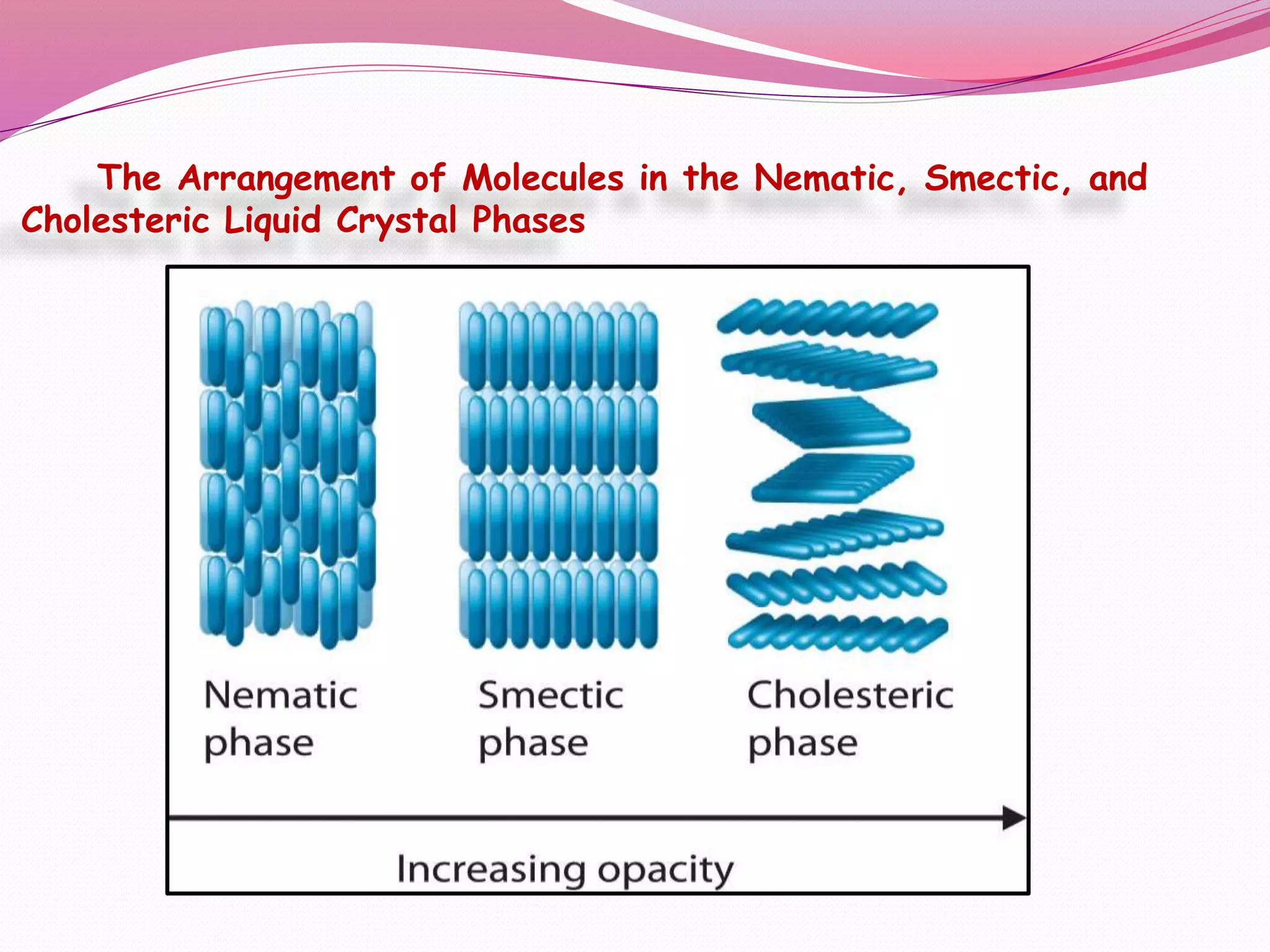
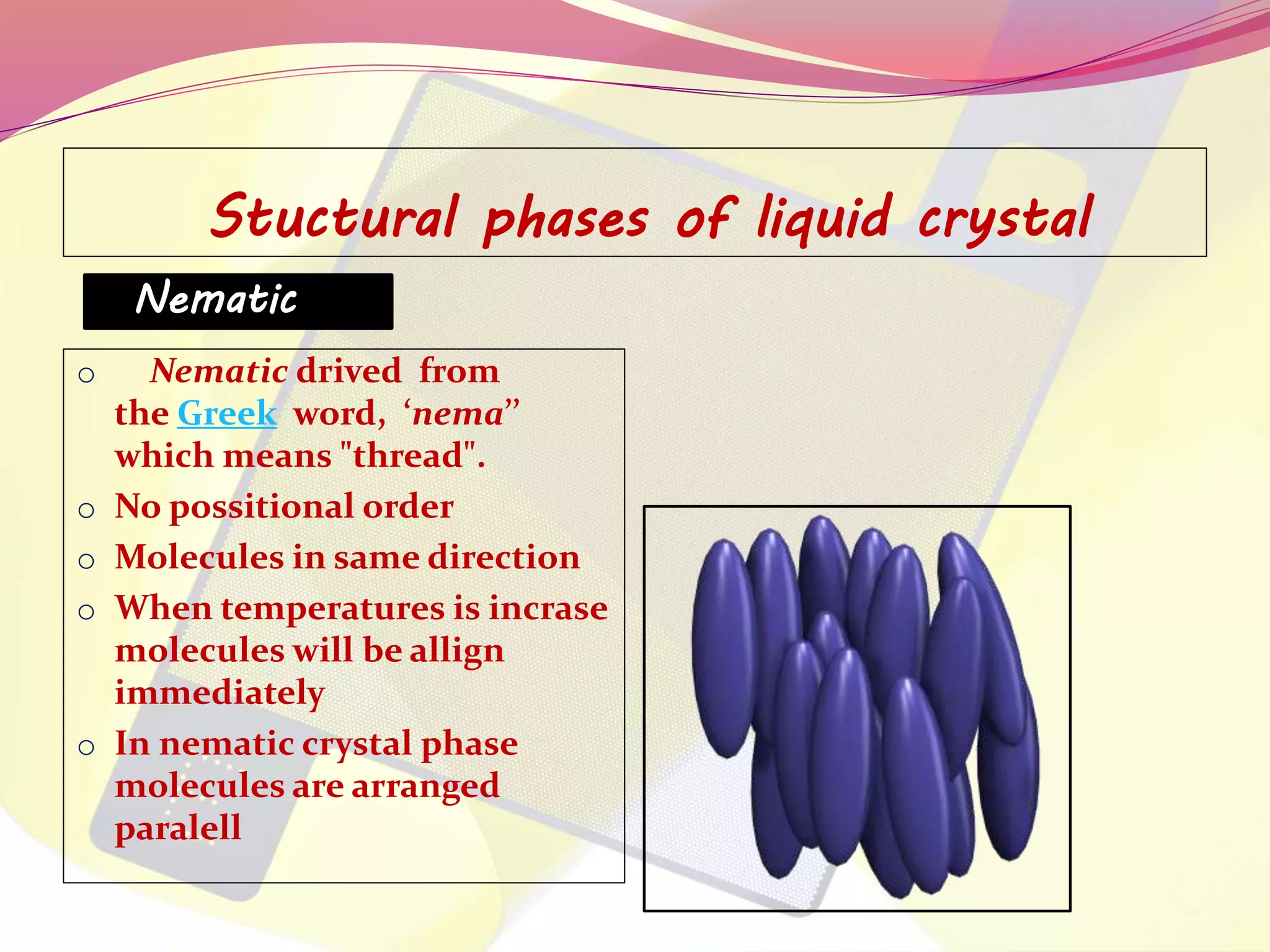
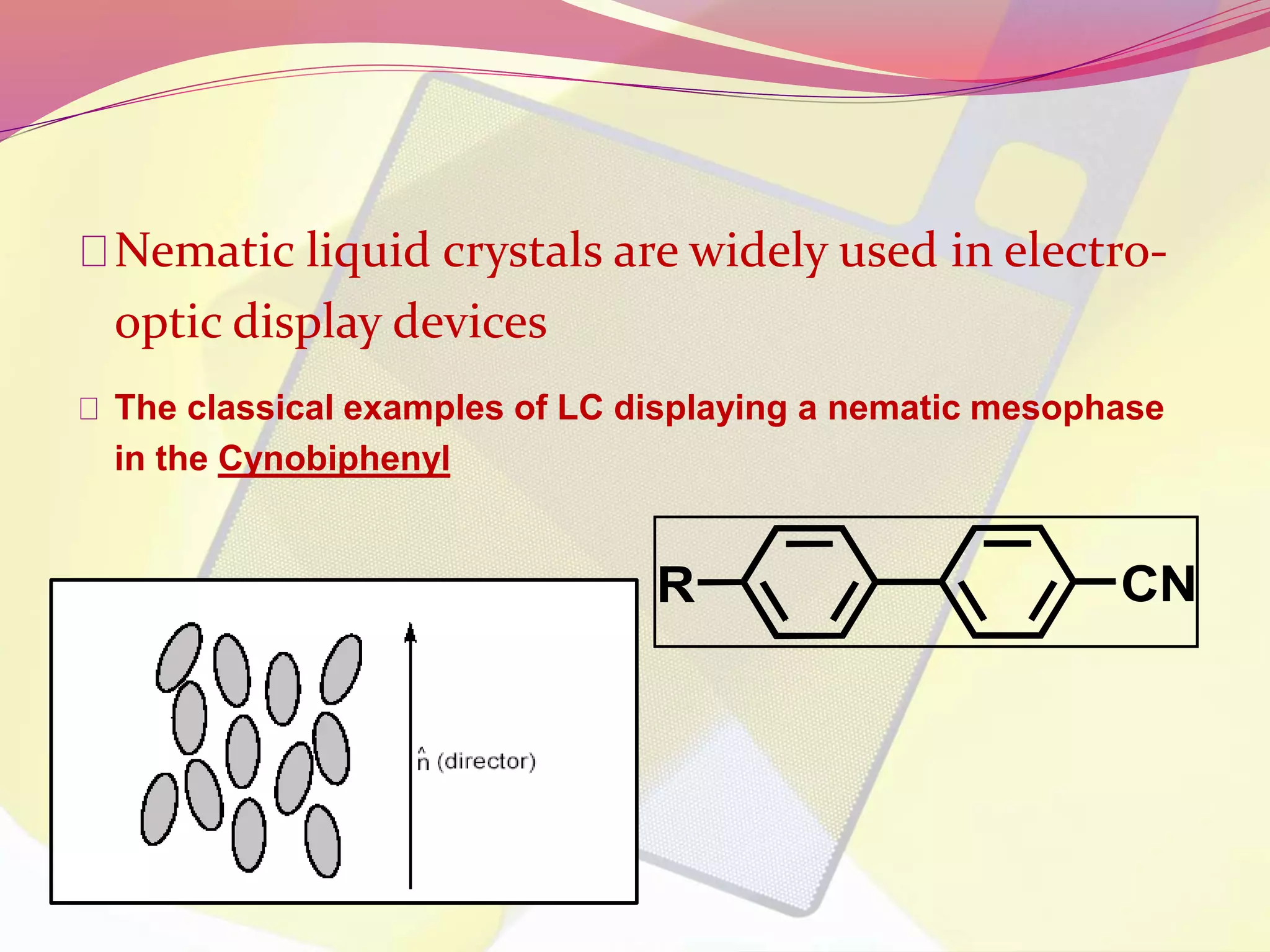
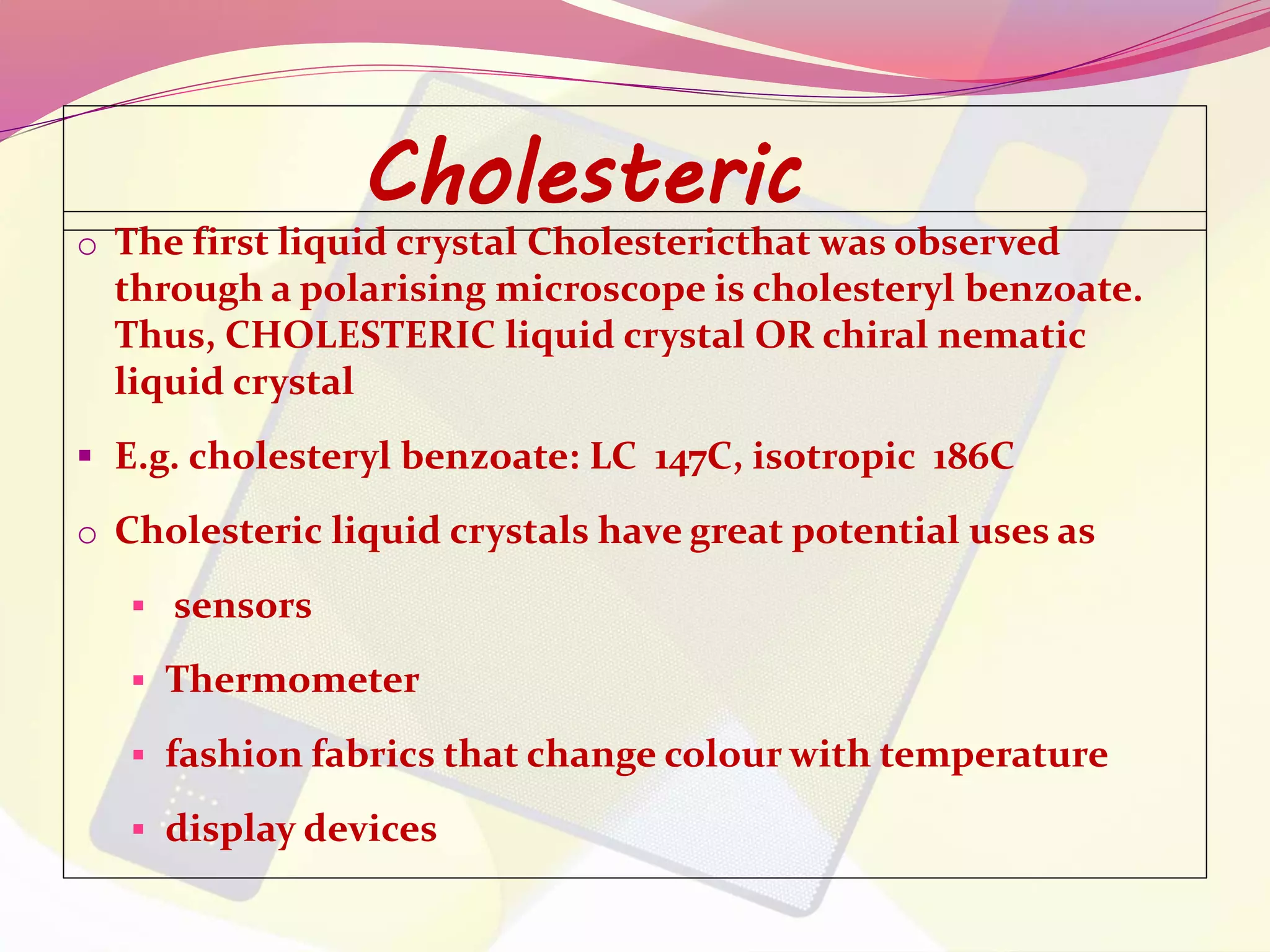
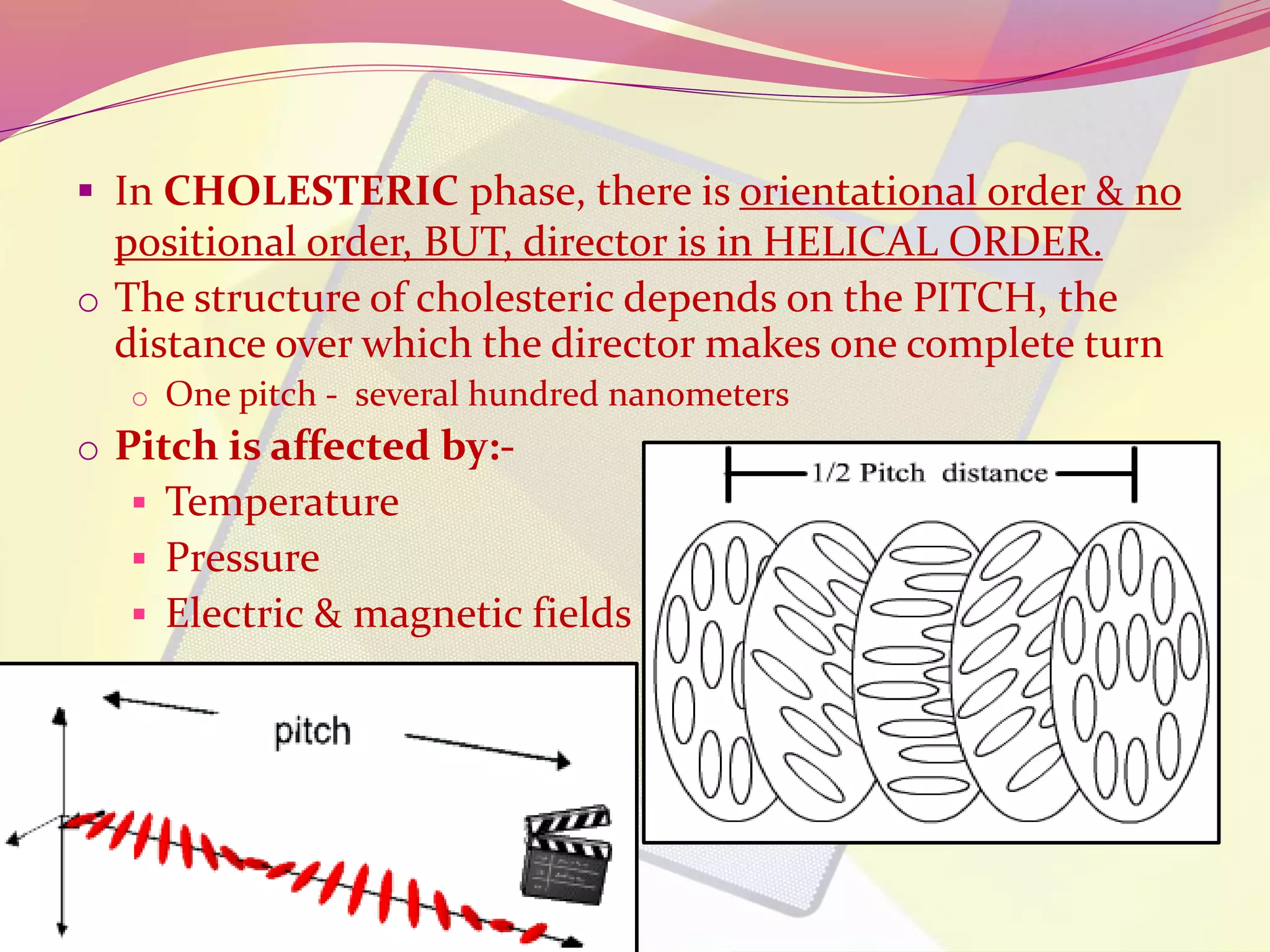
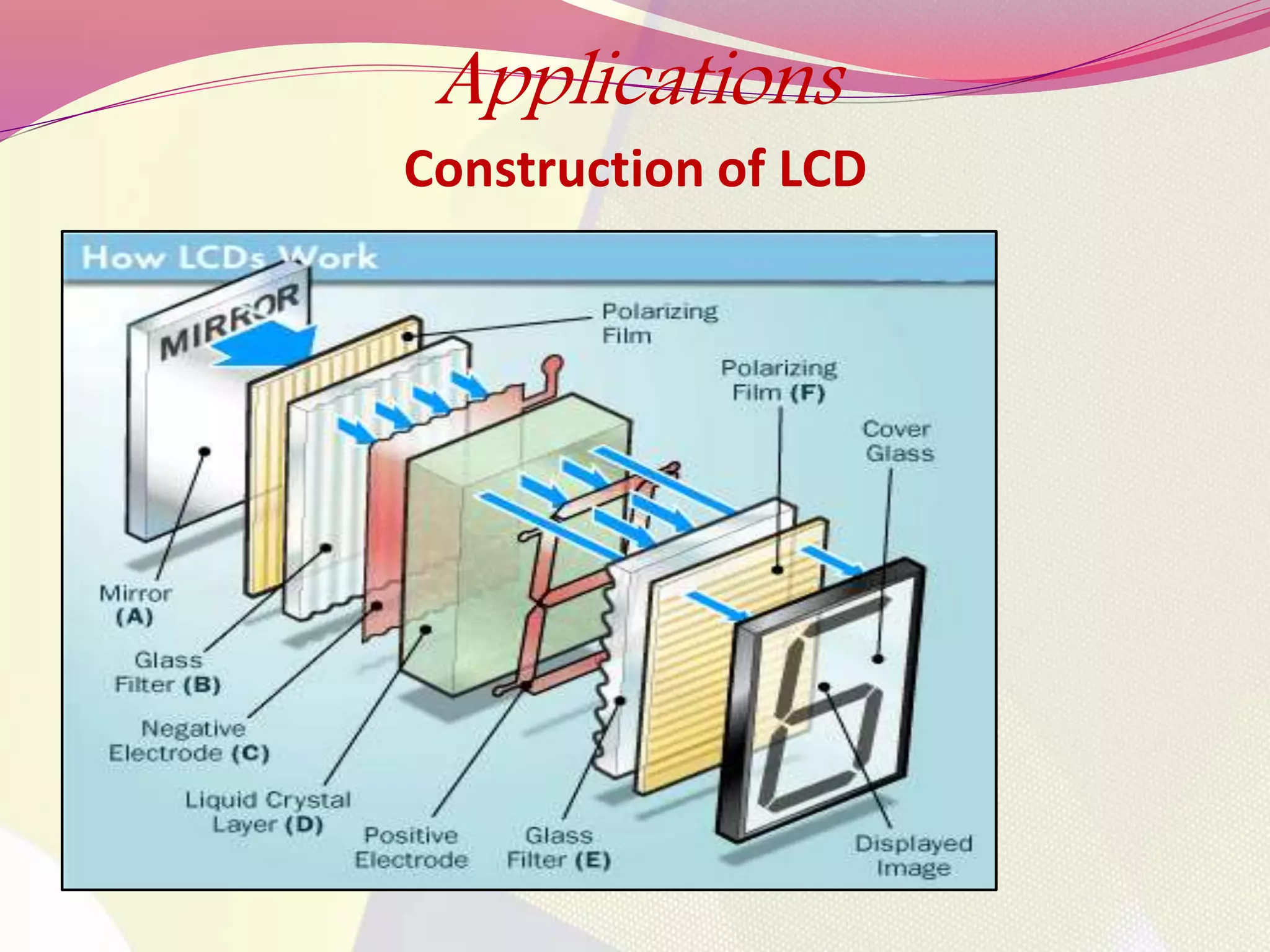
This document discusses liquid crystals, their properties, types, and applications. It describes how liquid crystals have properties between solids and liquids, with some degree of molecular order. The main types discussed are thermotropic and lyotropic liquid crystals. Thermotropic liquid crystals change phase based on temperature, while lyotropic crystals depend on temperature, concentration, and solvent. Common applications mentioned include digital watches, phones, displays, and electronic devices that take advantage of liquid crystals' response to electric fields.








![Smectic
o SMECTIC phase occurs at temperature
below nematic or cholesteric
o Molecules align themselves approx. parallel
& tend to arrange in layers
o Chiral smectic C liquid crystals are useful in
LCDS
Eample: 4,4’’’ – Bis-nonyloxy-[1,1’;1’’;4’’,1’’’]
quaterphenyl (2)](https://image.slidesharecdn.com/presentation1-140907234838-phpapp02/75/liquid-crystals-and-their-applications-29-2048.jpg)



![References
1: P.G de Gennes, Port.j , 2010 “The Physics of Liquid Crystals”, ref 5.
2: M. J. Stephen, Excellent review of basic properties
3: J. P. Straley “Physics of liquid crystals”, Ref. [2].
4: D. Fincham, rotational motion of linear molecules, 1984 , 47–48.
5: http://www.slideshare.net/Nawarajintermediate/liquid-crystal-and-its-application#
6: Stegemeyer H, Blumel T, Hiltrop K, Onusseit H and Porsch F, Liq. Cryst. 1986,1-28.
7: Tanimoto K and Crooker P,1985, Phys. Rev. A 32 1893-5.
8: Tsvetkov V, Acta Physicicochim (USSR) , 1942 (16- 132).
9: van der Meer B Wand Vertogen G, Phys. Lett, 1976. 59A( 279-81).
10: Wright D C and Mermin N D, Phys. Rev, 1985. A 31 3498-500.
11:Thoen J. 1988 Phys. Rev. A 37 1754-9
12 Tsvetkov V 1942 Acta Physicicochim (USSR) 16 132
13 Tanimoto K and Crooker P P 1984 Phys. Rev. A 29 1566-7 13 Tanimoto K, Crooker P P
and Koch G C 1985 Phys. Rev. A 32 1893-5](https://image.slidesharecdn.com/presentation1-140907234838-phpapp02/75/liquid-crystals-and-their-applications-34-2048.jpg)
