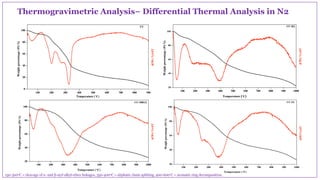
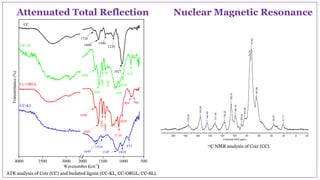
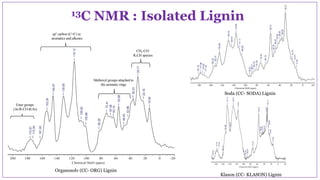
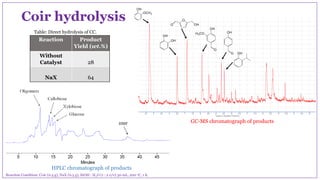
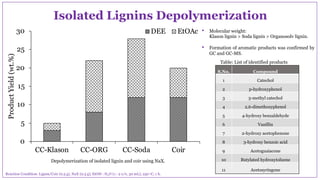
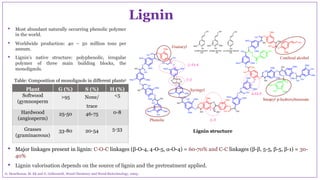
The document discusses the isolation, characterization, and depolymerization of lignin derived from coconut coir using solid base catalysts. It highlights the chemical composition of coir, methods for lignin extraction, and various characterization techniques, revealing that the Klason method yields the highest amount of lignin. Additionally, it presents findings on the effectiveness of solid base catalysts for lignin depolymerization at lower temperatures, achieving significant yields of aromatic products.


![Microanalysis
Microanalysis Coconut Coir
(CC)
Organosolv Lignin
(CC-ORGL)
Soda Lignin
(CC-SL)
Klason Lignin
(CC-KL)
C (%) 45.35 60.78 63.58 63.59
H (%) 4.86 5.41 6.08 6.12
O (%) 49.79 33.81 29.34 29.39
O/C 1.09 0.56 0.46 0.46
H/C 0.11 0.09 0.10 0.10
HHV (MJ/kg) (a) 13.4 22.3 24.8 24.9
DBE (b) 2.18 5.8 5.6 5.6
MMF (c) C7.6H8.84O6.22 C10.1H10.7O4.2 C10.6H12.1O3.8 C10.6H12.1O3.8
pH (d) 6.4 6.3 5.9 2.95
(a) Higher heat value (HHV) = [0.3383 x C + 1.442 x [H-(O/8)] + 9.248 x S] where C, H, O and S are wt.% of carbon, hydrogen, oxygen and sulphur; (b) Double bond
equivalence (DBE) = [C – (H/2) + (N/2) + 1] where C, H and N are number of carbon, hydrogen and nitrogen atoms found from monomer molecular formula (c)
Monomer
molecular formula (MMF) = 100 - (‘C’ wt.% + ‘H’ wt.% + ‘O’ wt.%). (d)
pH was measured by dissolving 0.08 g sample in 5 mL water
Characterizations: Coir & Isolated Lignin](https://image.slidesharecdn.com/ligninisolationfromcoconutcoircharacterizationanddepolymerizationusingsolidbasecatalyst-190513075855/85/Lignin-isolation-from-coconut-coir-characterization-and-depolymerization-using-solid-base-catalyst-5-320.jpg)