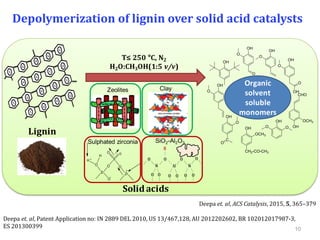
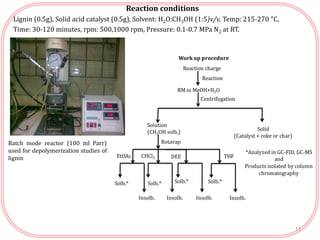
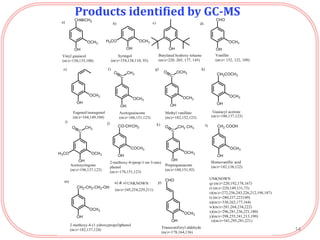
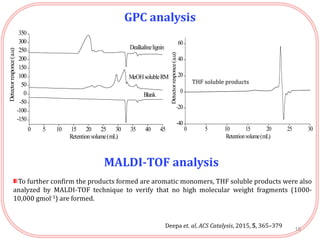
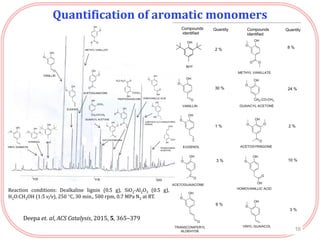
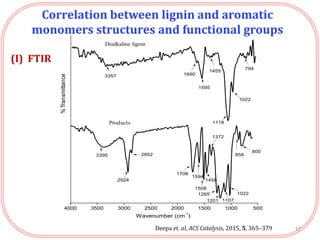
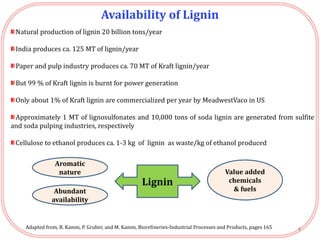
The document summarizes research on the depolymerization of lignin over heterogeneous catalysts having acidic functionality. Key points:
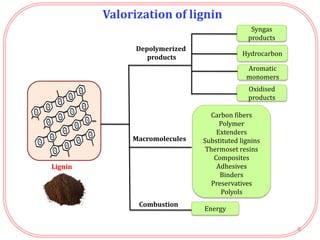
- Lignin can be converted into high-value chemicals but is currently underutilized. Heterogeneous catalysts can aid in the depolymerization of lignin into aromatic monomers.
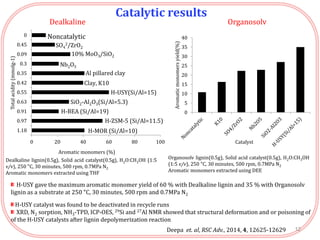
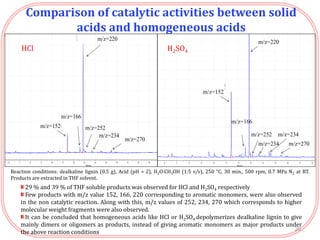
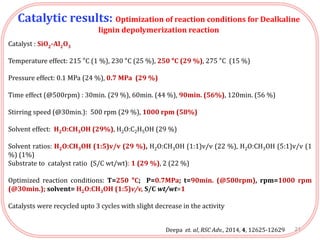
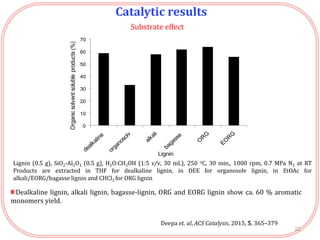
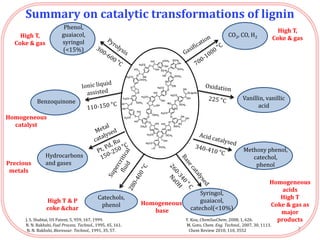
- Testing of various solid acid catalysts showed H-USY zeolite gave the highest yield (60%) of aromatic monomers from depolymerization of dealkaline lignin. However, H-USY deactivated after reuse.
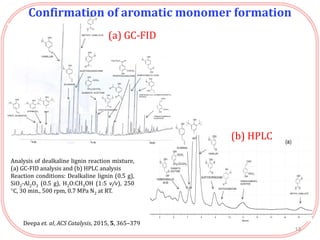
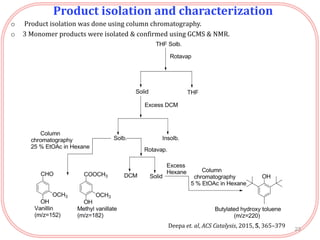
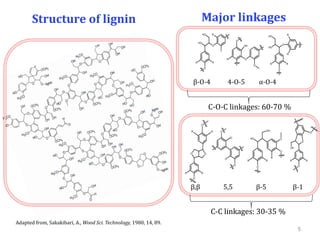
- Products were analyzed using GC-MS, GPC and MALDI-TOF, confirming the formation of aromatic monomers rather than high molecular weight compounds. FTI

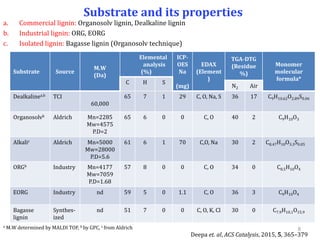
![Properties of solid acids (for lignin depolymerization)
aBrunauer–Emmett–Teller surface area, bPorevolume, bPorediameter [Autosorb1C Quantachrome, instrument]
dAcidity measured by means of TPD of NH3[Micrometrics Autochem-2910 instrument] .
Catalyst Structure
Nitrogen sorption NH3-TPDd
BET SAa
(m2g-1)
Vb
(cm3g-1)
Dc
(nm)
Weak acid
sites
(mmolg-1)
Stong acid
sites
(mmolg-1)
Total
acidity
(mmolg-1)
H-USY (Si/Al=15) Micro 873 0.45 0.61 0.06 0.49 0.55
H-ZSM-5 (Si/Al=11.5) Micro 423 0.22 0.60 0.37 0.61 0.97
H-BEA (Si/Al=19) Micro 761 0.34 0.60 0.25 0.66 0.91
H-MOR (Si/Al=10) Micro 528 0.22 0.59 0.5 0.65 1.18
Nb2O5 -- 115 -- -- 0.30 -- 0.30
SO42-/ZrO2 -- 84 0.02 -- nd nd nd
Clay (K10) Layered 246 0.3 -- 0.09 0.33 0.42
Al pillared clay Layered nd nd nd nd nd nd
SiO2-Al2O3 Micro-meso 532 0.82 4.90 0.17 0.46 0.63
10%MoO3/SiO2 Nonporous nd nd nd 0.09 - 0.09
9](https://image.slidesharecdn.com/lignin-solidacid-heterogeneouscatalyst-depolymerization-aromaticmonomer-151102084152-lva1-app6892/85/Lignin-depolymerization-aromatic-monomers-solid-acid-heterogeneous-catalyst-A-K-Deepa-Paresh-Dhepe-NCL-9-320.jpg)