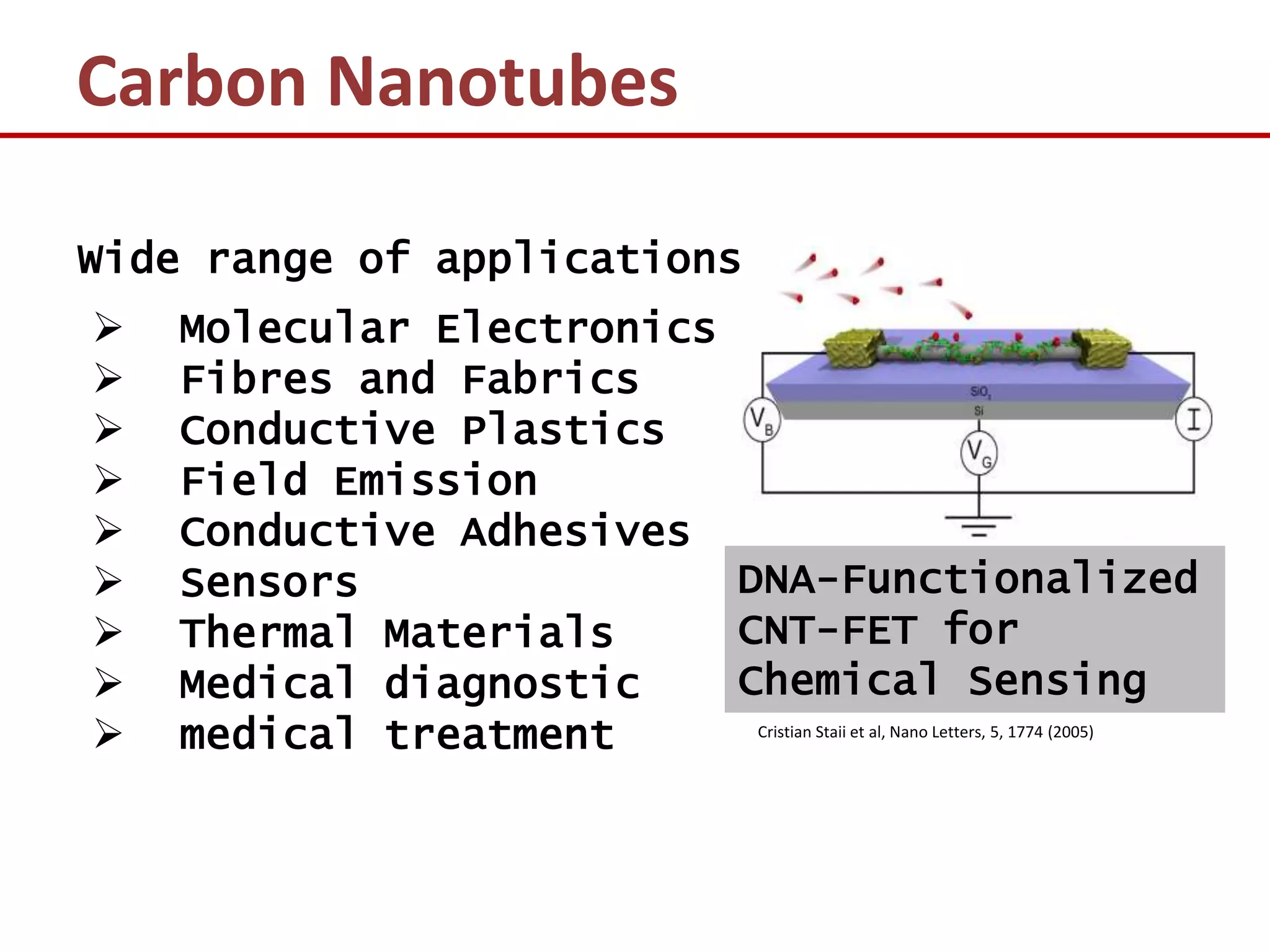
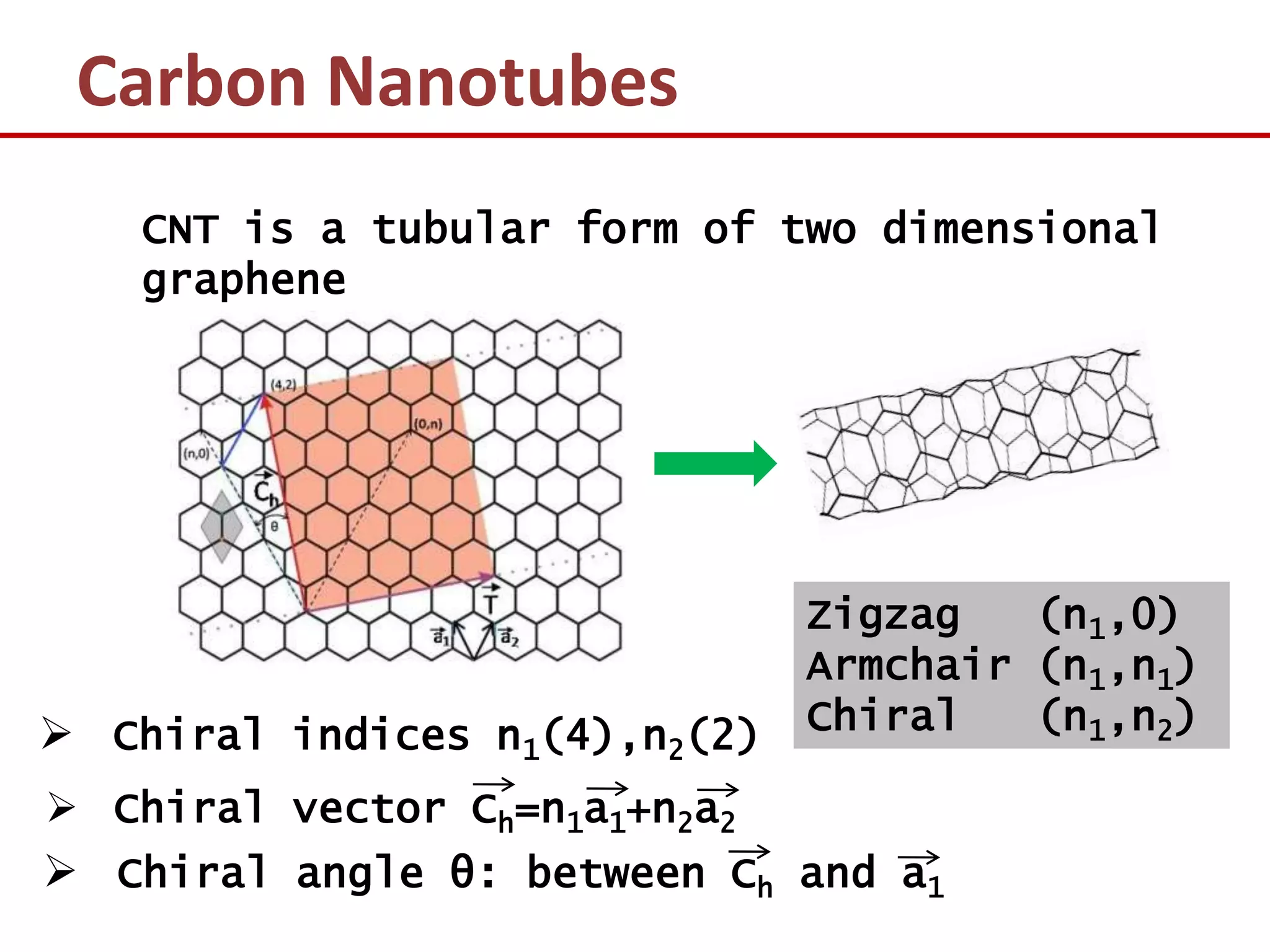
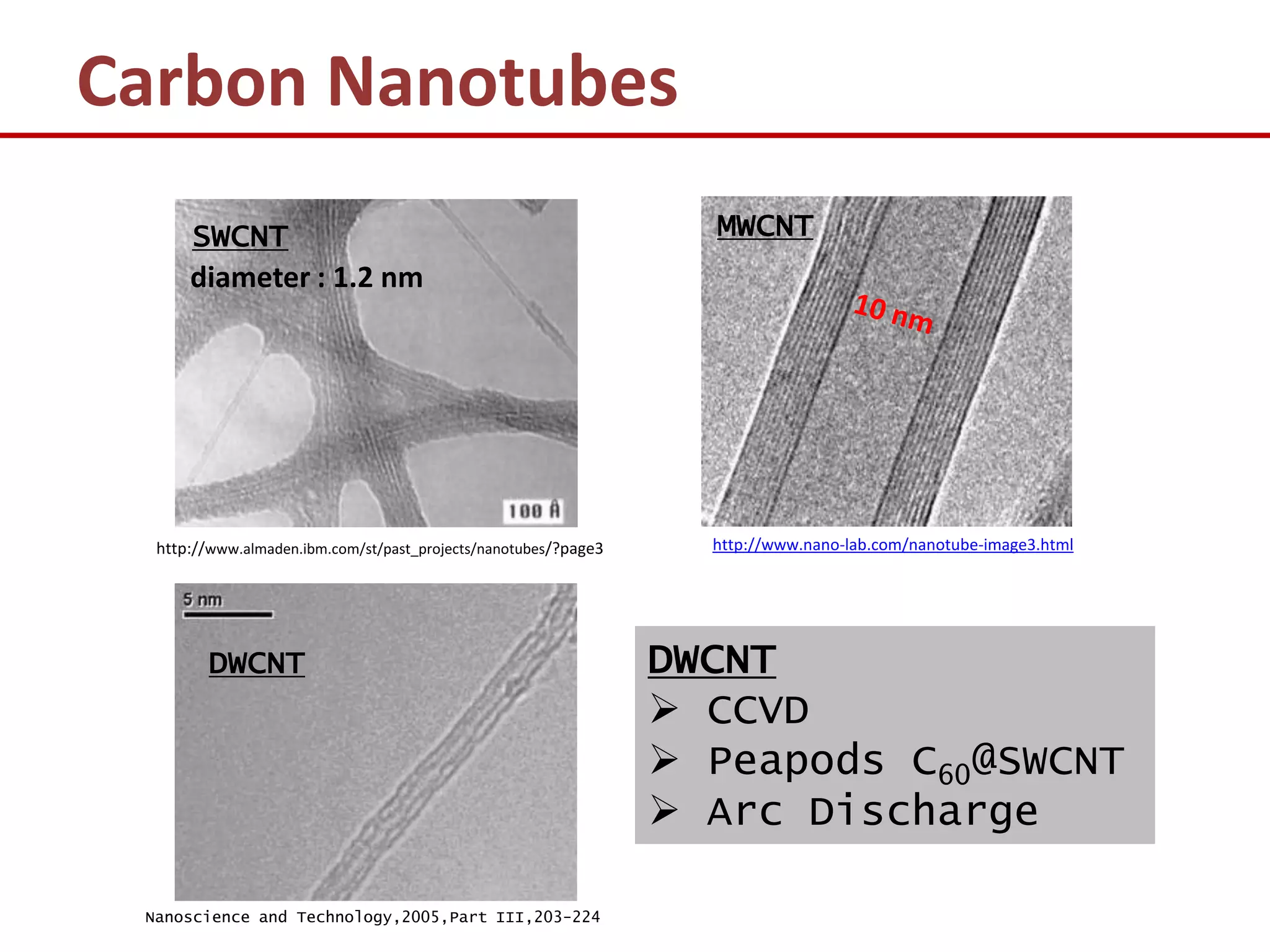
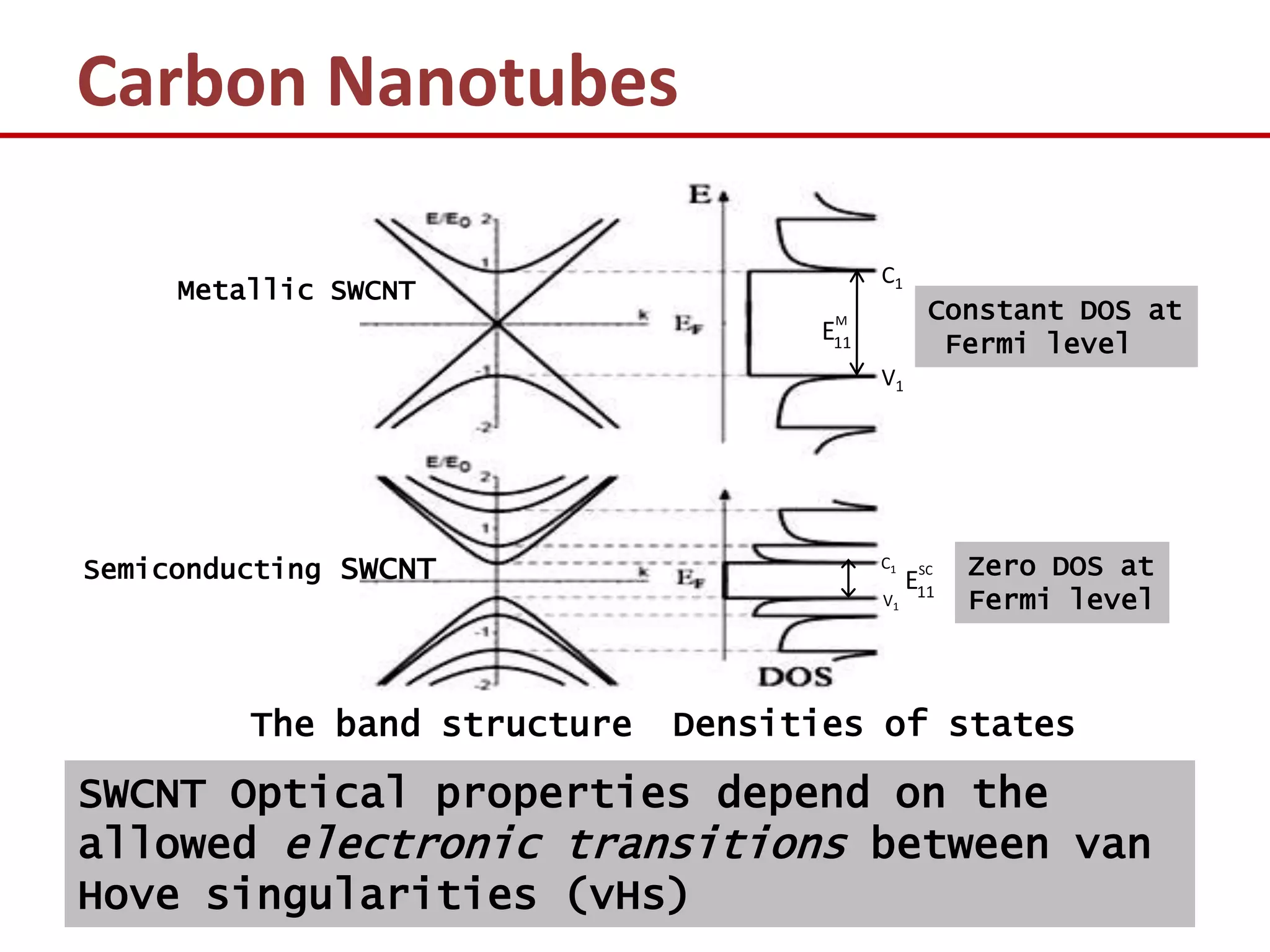
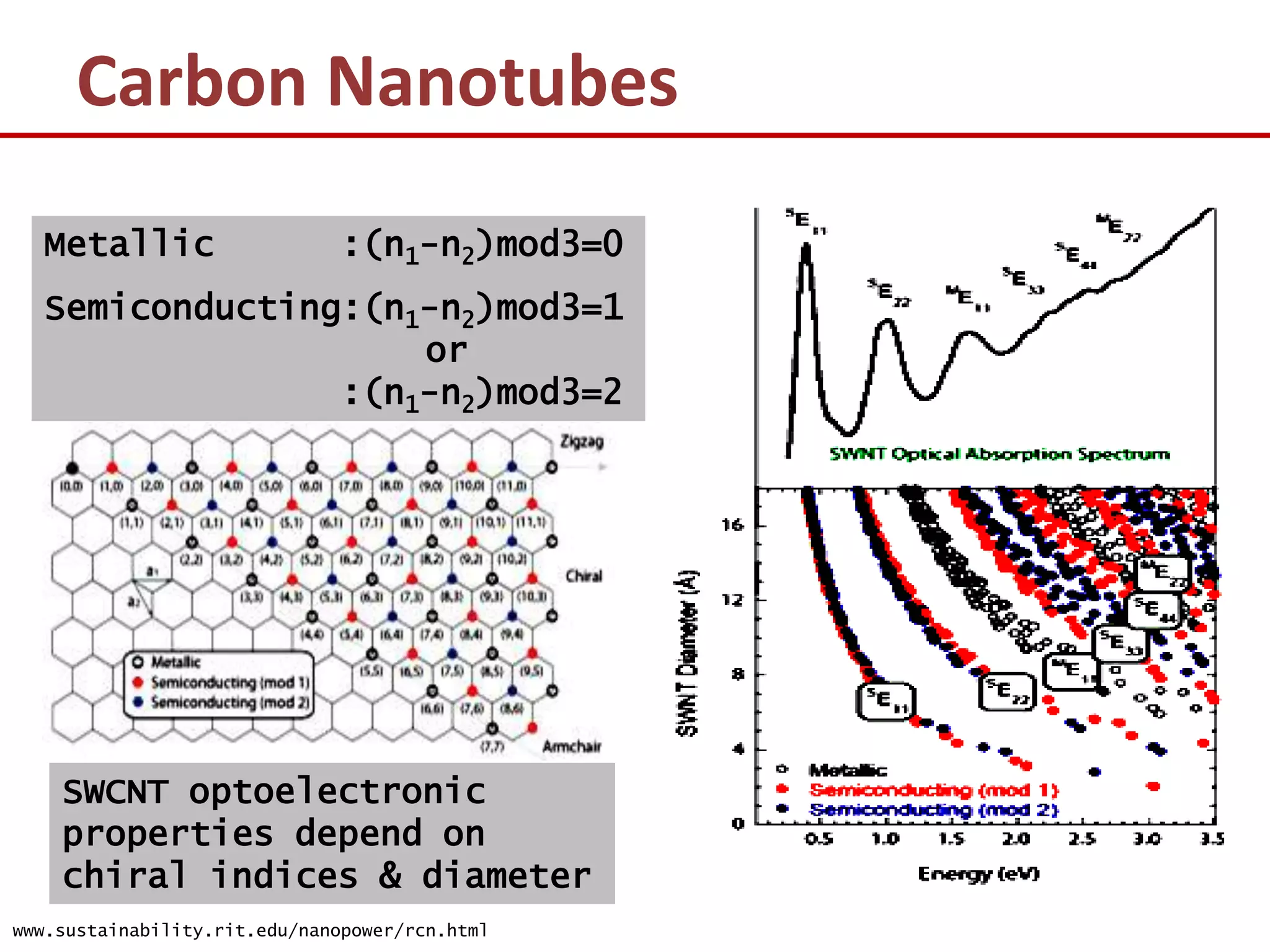
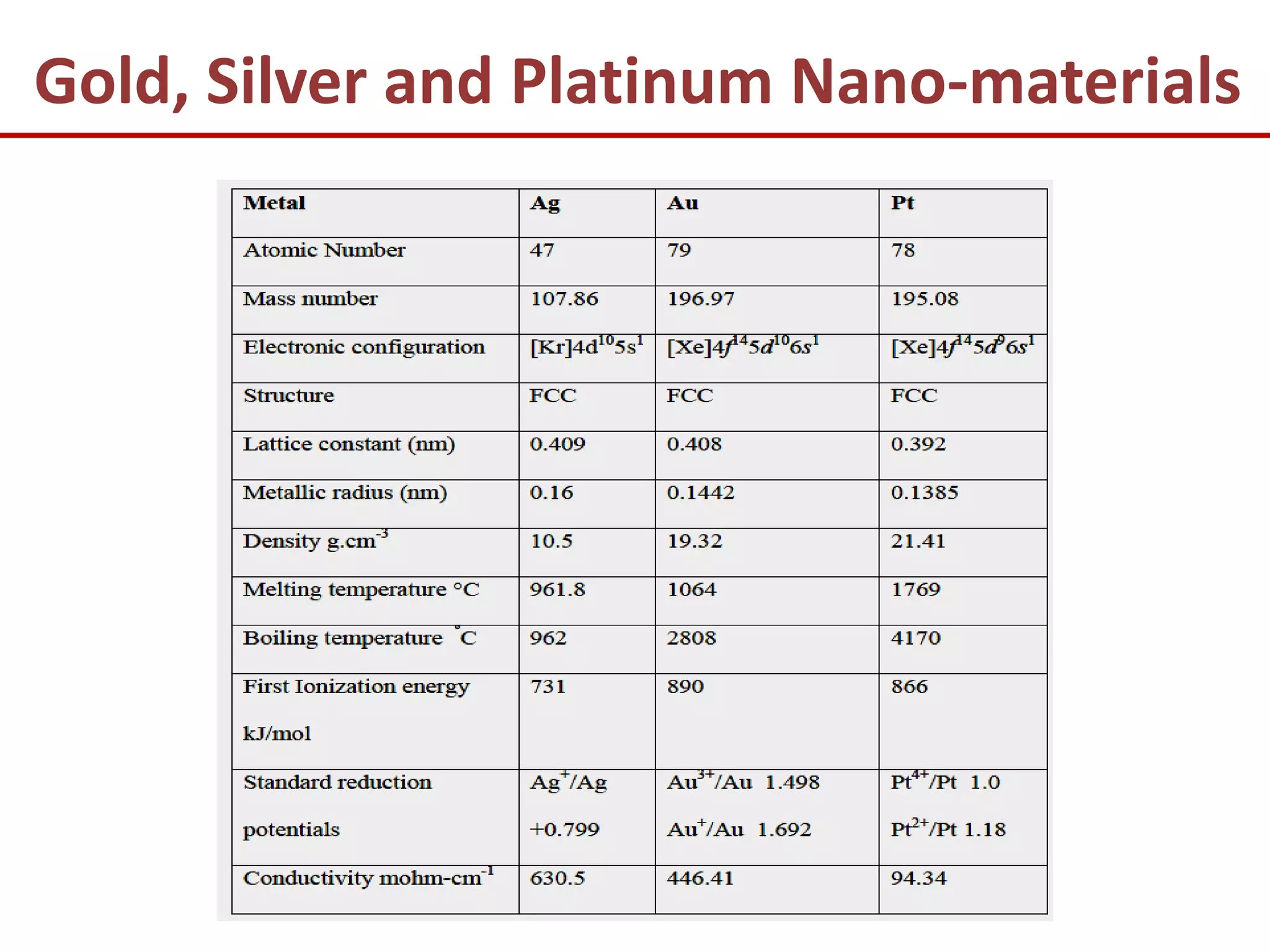
The document provides an introduction to nano-science and nano-technology. It discusses several key topics:
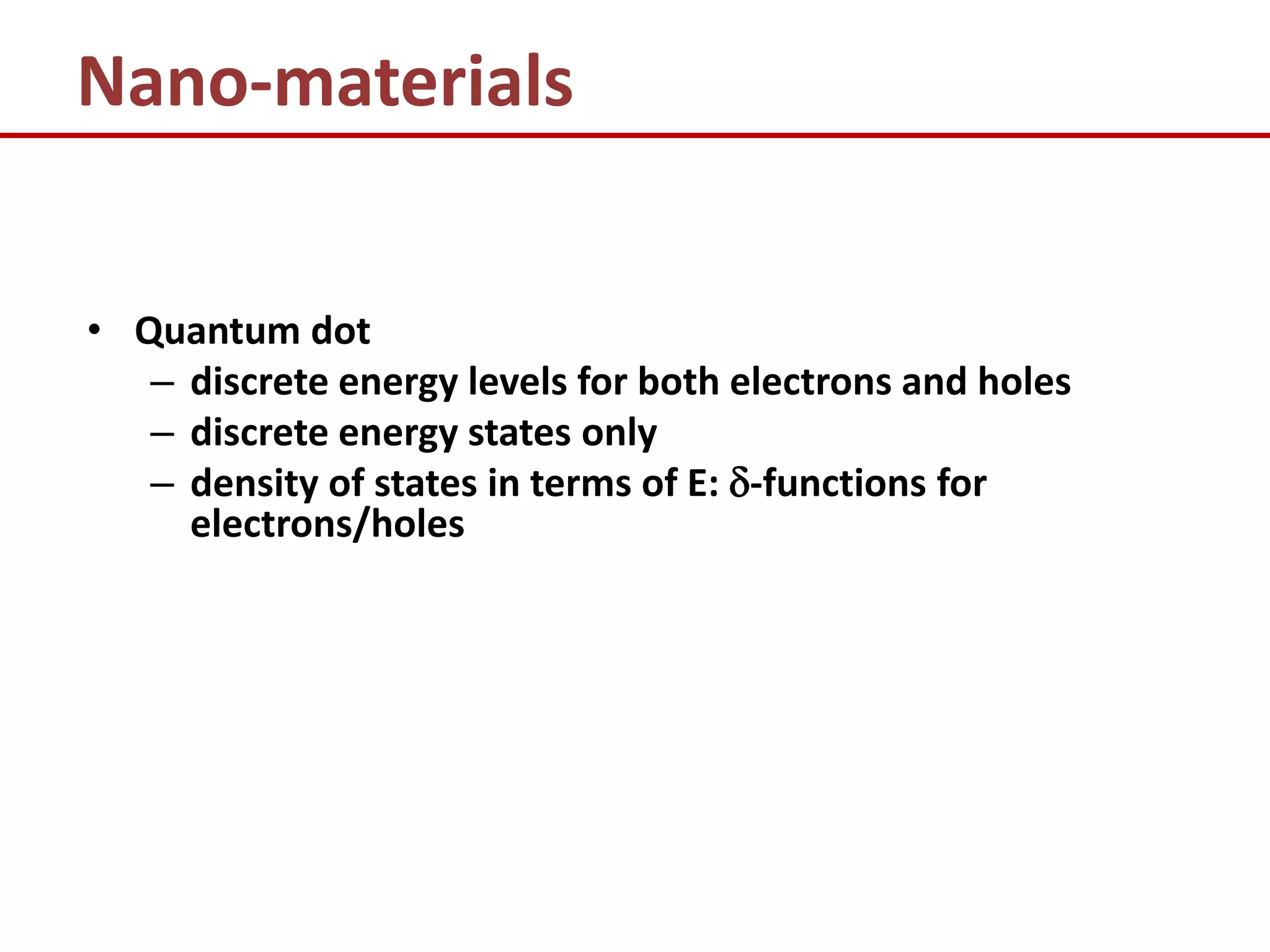
1) It defines nano-materials as low dimension structures like quantum wells, wires, and dots, which can exhibit different electronic and optical properties than bulk materials due to electron confinement effects.
2) It explains how the behavior of electrons is affected by the level of confinement in various nanostructures like quantum wells, wires, and dots.
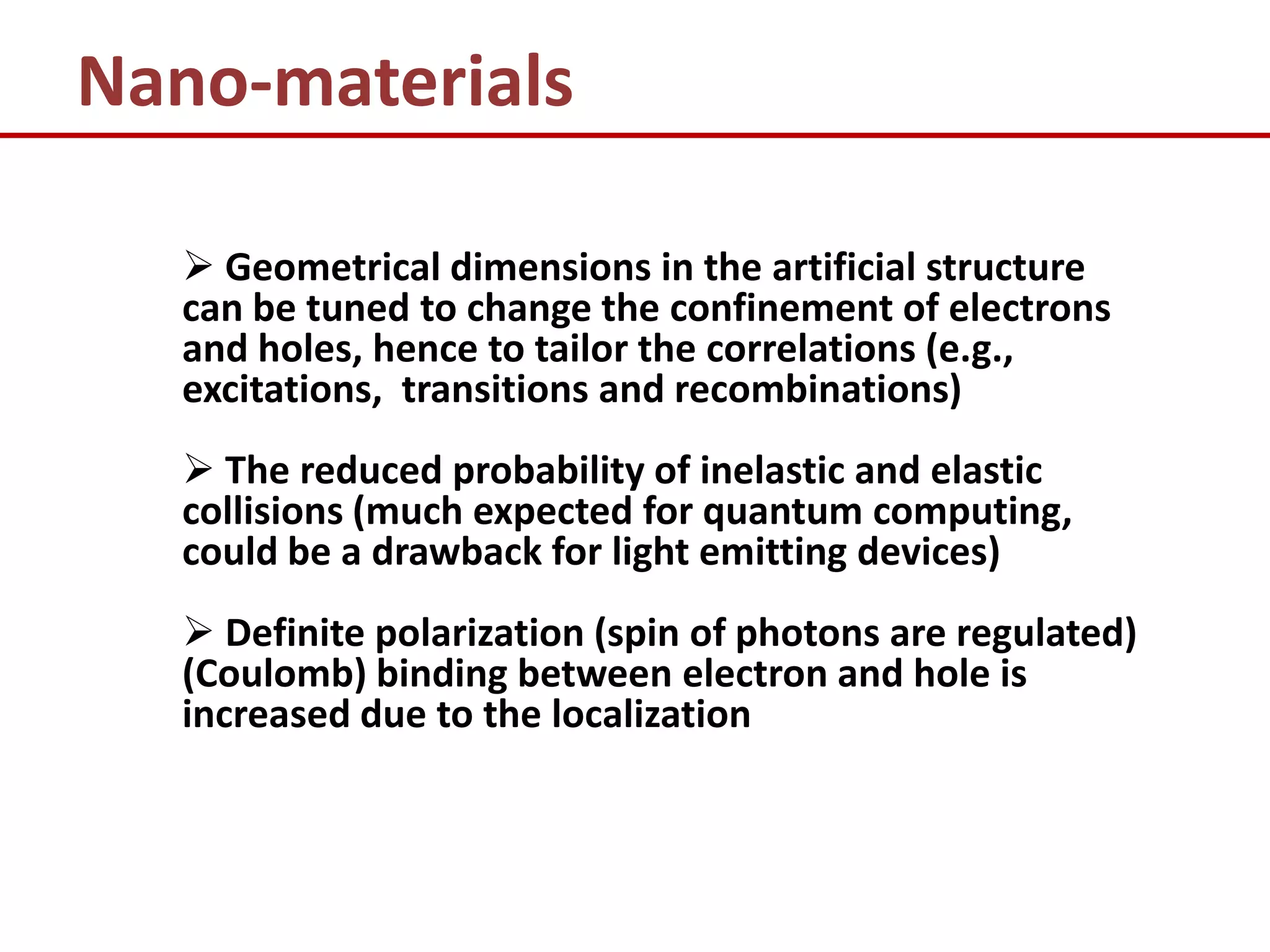
3) It discusses how nanostructures allow for tailoring of electronic and optical correlations through control of geometric dimensions and electron confinement.