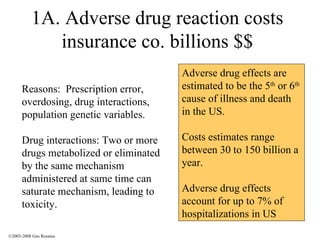
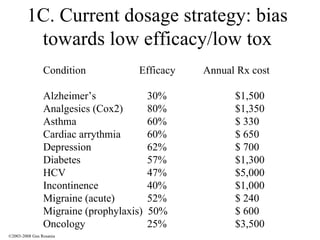
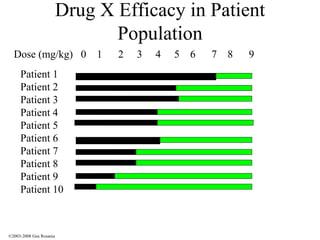
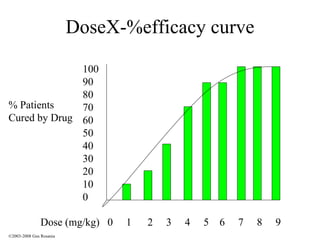
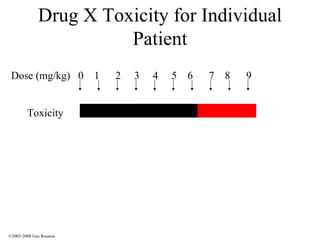
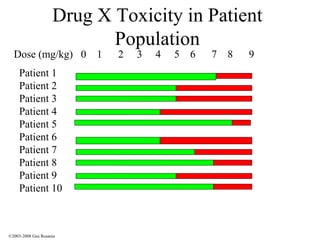
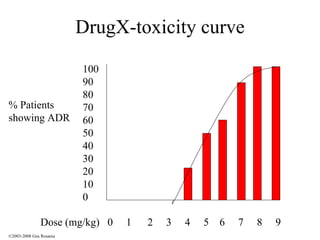
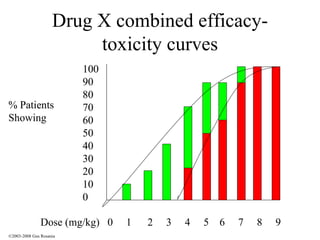
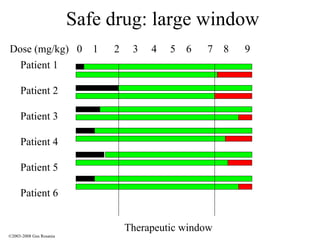
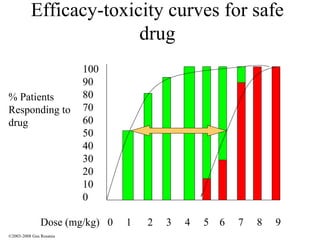
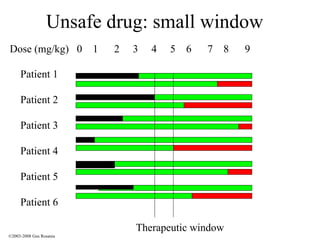
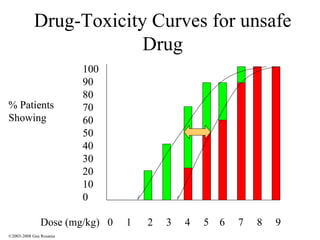
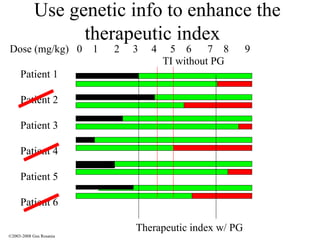
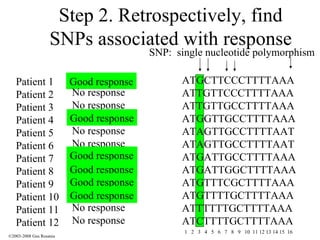
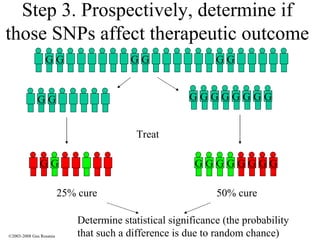
The document discusses the principles of pharmacogenomics. It defines pharmacogenomics as using genetic information to predict drug response. The key forces driving pharmacogenomics in healthcare are reducing adverse drug reactions and drug development costs. There are four steps to translating pharmacogenomic research to practice: 1) identify genetic variants related to drug response, 2) associate variants with past responses, 3) validate prospective impact on outcomes, and 4) require genetic testing before treatment.