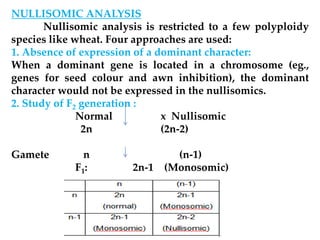
This document discusses polyploidy in plant breeding. It defines key terms like diploidy, polyploidy, haploidy, and aneuploidy. It describes different types of polyploids like autopolyploids which have identical genomes, and allopolyploids which have distinct genomes. Methods to produce polyploids like colchicine treatment are explained. The document also discusses applications of aneuploids, autopolyploids and allopolyploids in crop improvement through techniques like trisomic analysis and chromosome substitution. Both advantages and limitations of polyploidy in plant breeding are summarized.