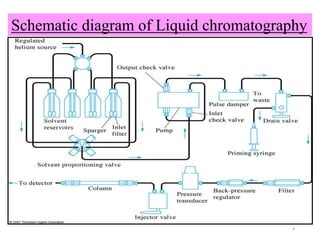
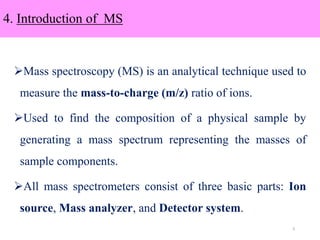
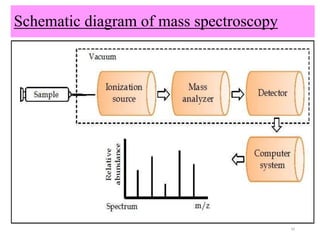
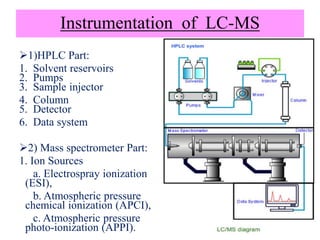
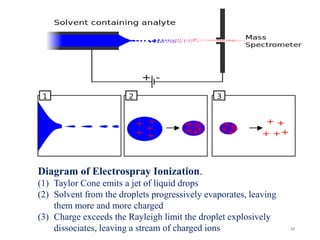
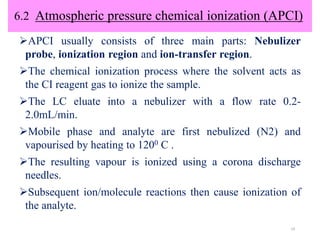
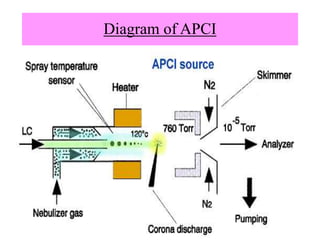
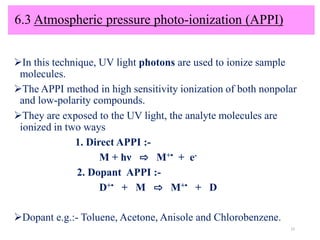
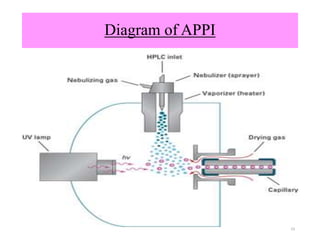
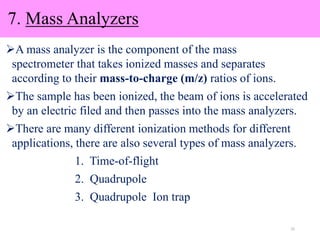
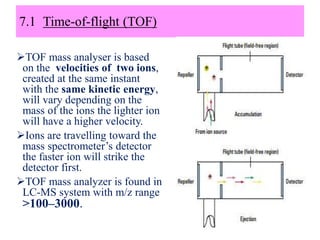
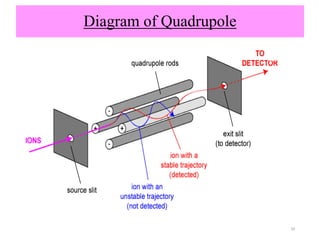
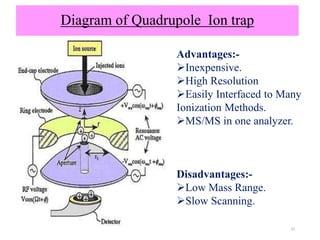
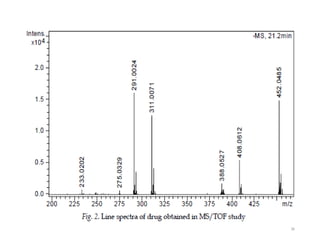
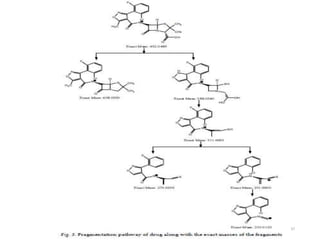
The document discusses liquid chromatography-mass spectrometry (LC-MS), a hyphenated technique that combines liquid chromatography with mass spectrometry. It describes the basic components and workings of LC, MS, and LC-MS. Key interfaces for LC-MS coupling include electrospray ionization, atmospheric pressure chemical ionization, and atmospheric pressure photoionization. Common mass analyzers are quadrupoles, ion traps, and time-of-flight analyzers. The document outlines applications of LC-MS such as drug discovery, food analysis, and environmental and biomedical studies.

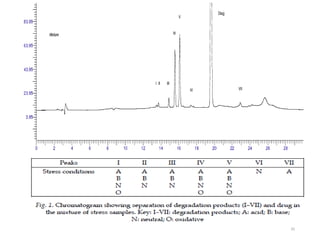
![1. Degradation Analysis
To help to identify reactions that cause degradation of
pharmaceutical product.
The stability of a drug product or a drug substance is a critical
parameter which may affect purity, potency and safety.
Changes in drug stability can risk patient safety by formation of
a toxic degradation products or deliver a lower dose than
expected.
Knowledge of the stability of molecule helps in selecting proper
formulation and packaging.
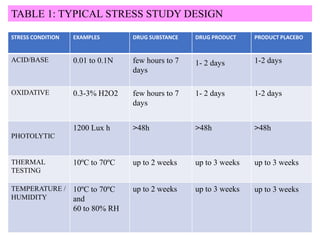
The various type of Degradation
1. Hydrolysis [Acidic, Alkaline and Neutral]
2. Oxidation
3. Photolytic
4. Thermal 3](https://image.slidesharecdn.com/degradationanalysisusinglc-msms-170816094605/85/Degradation-Analysis-Using-LC-MS-MS-3-320.jpg)
![This two Guideline are used for stability testing:-
1. ICH Q1A(R2) [Stability Testing of New Drug Substances
and Products]
2. ICH Q1B [Photostability Testing of New Active
Substances and Medicinal Products]
Degradation studies are carried out for the following
reasons:
To solve stability-related problems.
To generate more stable formulation.
To identify impurities related to drug substances.
To develop and validate a stability indicating method.
To determine degradation pathways of drug substances and
drug products.
4](https://image.slidesharecdn.com/degradationanalysisusinglc-msms-170816094605/85/Degradation-Analysis-Using-LC-MS-MS-4-320.jpg)