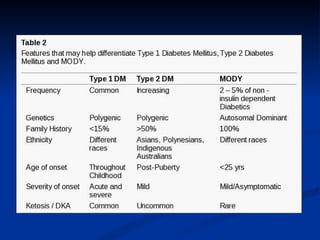
MODY, or Maturity-Onset Diabetes of the Young, is a form of diabetes that is caused by single-gene mutations. It is characterized by an onset of diabetes early in life, often before age 25, and autosomal dominant inheritance. There are several subtypes of MODY based on the gene involved, including MODY1-6. MODY often presents with mild, stable hyperglycemia that does not progress rapidly and may initially respond to oral medications rather than insulin injections. Genetic testing can confirm a MODY diagnosis but is not necessary as clinical features are also diagnostic. Management depends on the specific gene mutation but usually involves diet, exercise and oral medications long-term.





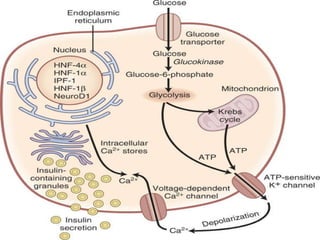
![MODY-Related Proteins [1/4] Glucokinase Expressed in -cells and liver GSK catalyzes the formation of glucose-6-phosphate from glucose. Beta cells - “Glucose sensor” Control rate of Glucose phosphorylation Liver – Helps in storage of glucose as glycogen Mild stable hyperglycemia Does not respond to Sulfonylureas.](https://image.slidesharecdn.com/ladamodydiabetes-120213091400-phpapp01/85/LADA-MODY-DIABETES-7-320.jpg)

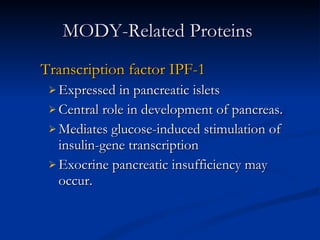
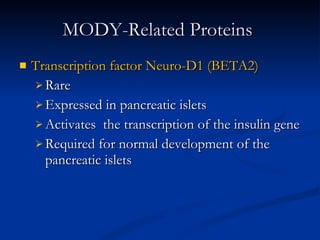















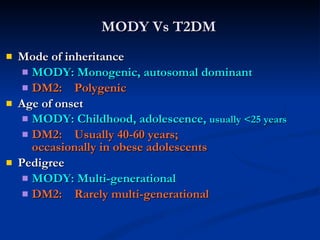
![Distinguishing Clinical Characteristics of MODY and Type 2 Diabetes [2/2] Penetrance MODY: 80-95 % DM2: Variable (10-40 %) Body habitus MODY: Not obese DM2: Usually obese Dysmetabolic syndrome MODY: Absent DM2: Usually present](https://image.slidesharecdn.com/ladamodydiabetes-120213091400-phpapp01/85/LADA-MODY-DIABETES-27-320.jpg)