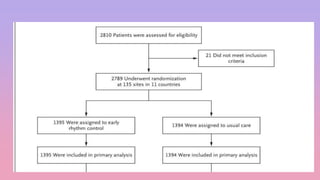
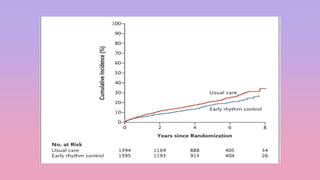
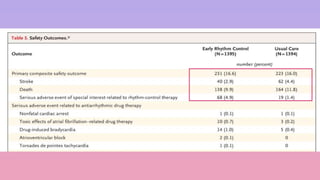
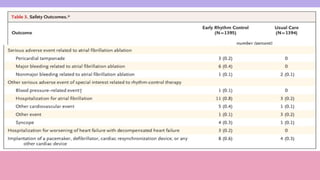
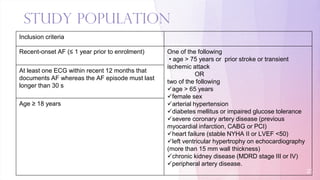
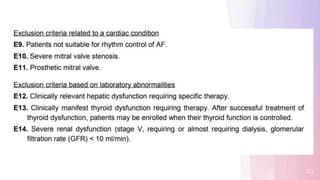
1) The EAST-AFNET trial compared an early rhythm control strategy to usual care for patients with recent-onset atrial fibrillation.
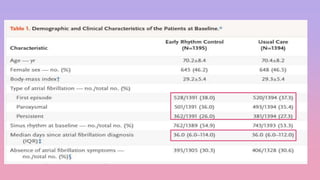
2) The early rhythm control strategy involved early use of antiarrhythmic drugs or ablation to maintain sinus rhythm, while usual care followed guidelines.
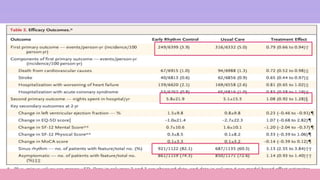
3) The trial was stopped early as early rhythm control reduced the composite outcome of death, stroke, or hospitalization compared to usual care over 5 years of follow-up.




![ first and largest study to compare rate-control and rhythm-control
strategies for the treatment of AF.
4,060 patients with non-valvular AF and a high risk of stroke or death
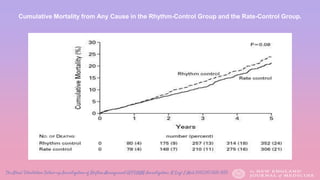
AFFIRM demonstrated no survival advantage between rhythm-
control and rate-control (using ß-blocker, calcium channel blocker
and/or digoxin) strategies. (mortality at five years, 23.8 percent and
21.3 percent, respectively; hazard ratio, 1.15 [95 percent confidence
interval, 0.99 to 1.34]; P=0.08).)
All patients were anticoagulated on warfarin initially, but patients in the
rhythm control arm who maintained normal sinus rhythm for at least
4 consecutive weeks could stop.
8](https://image.slidesharecdn.com/journalclubaf-201015150603/85/Journal-club-af-8-320.jpg)


![ a multicenter trial to evaluate the use of dronedarone in 4628 patients with atrial fibrillation who
had additional risk factors for death
Dronedarone reduced the incidence of hospitalization due to cardiovascular events or death in
patients with atrial fibrillation
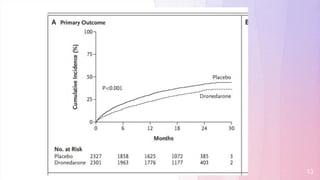
The primary outcome (first hospitalization due to cardiovascular events or death) occurred in 734
patients (31.9%) in the dronedarone group and in 917 patients (39.4%) in the placebo group, with a
hazard ratio for dronedarone of 0.76 (95% confidence interval [CI], 0.69 to 0.84; P<0.001).
The dronedarone group had higher rates of bradycardia, QT-interval prolongation, nausea, diarrhea,
rash, and an increased serum creatinine level than the placebo group. Rates of thyroid- and
pulmonary-related adverse events were not significantly different between the two groups.
12](https://image.slidesharecdn.com/journalclubaf-201015150603/85/Journal-club-af-12-320.jpg)












![Secondary outcomes reported here include
each component of the first primary outcome (analyzed in a time-
to-event analysis)
Rhythm
left ventricular function
quality of life, assessed with
European Quality of Life–5 Dimensions [EQ-5D]
visual analogue scale
12-Item Short-Form General Health Survey [SF-12])
atrial fibrillation–related symptoms (assessed as the European Heart
Rhythm Association [EHRA] score)
cognitive function (based on the Montreal Cognitive Assessment
[MoCA]) at 2 years](https://image.slidesharecdn.com/journalclubaf-201015150603/85/Journal-club-af-28-320.jpg)