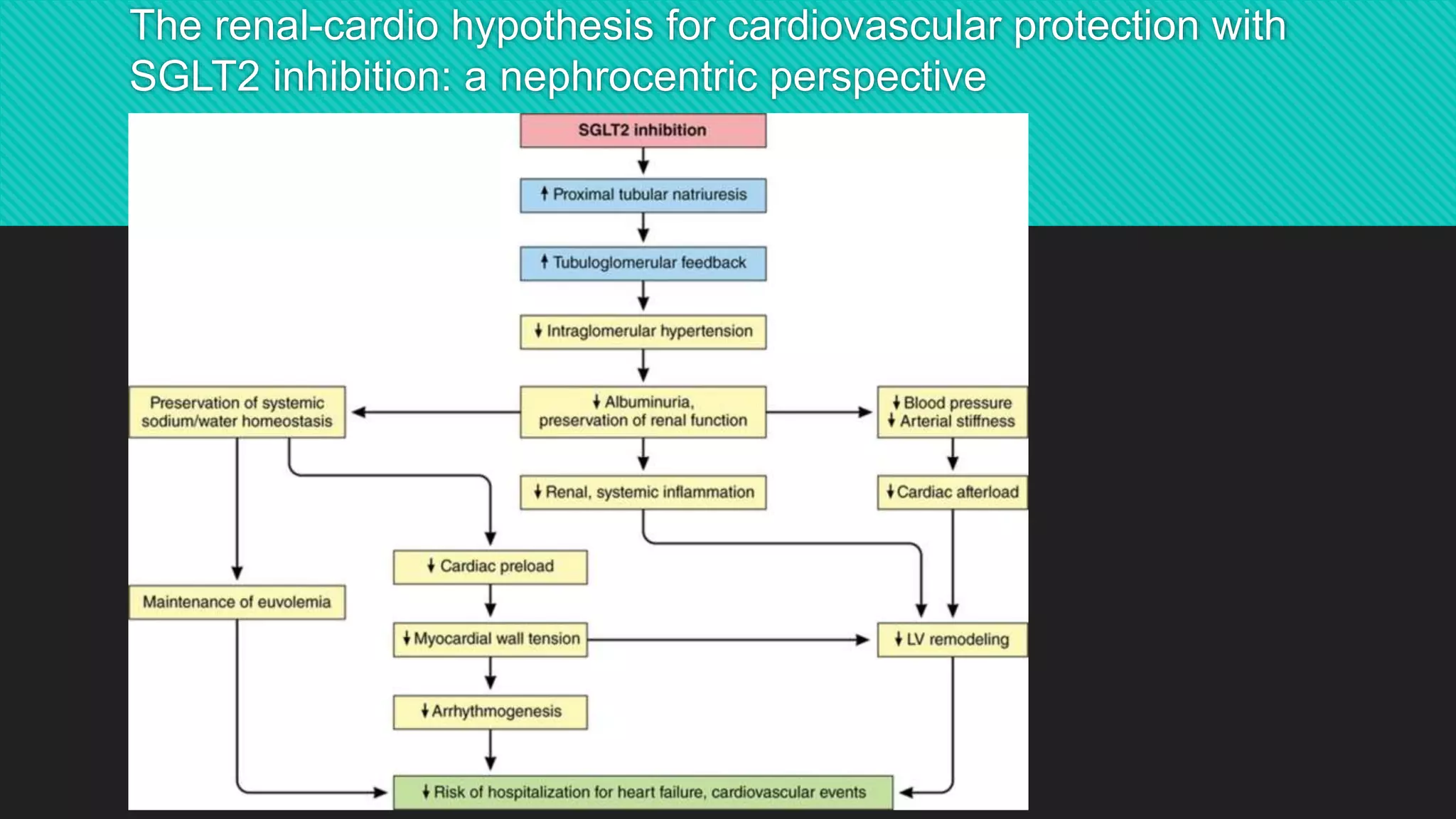
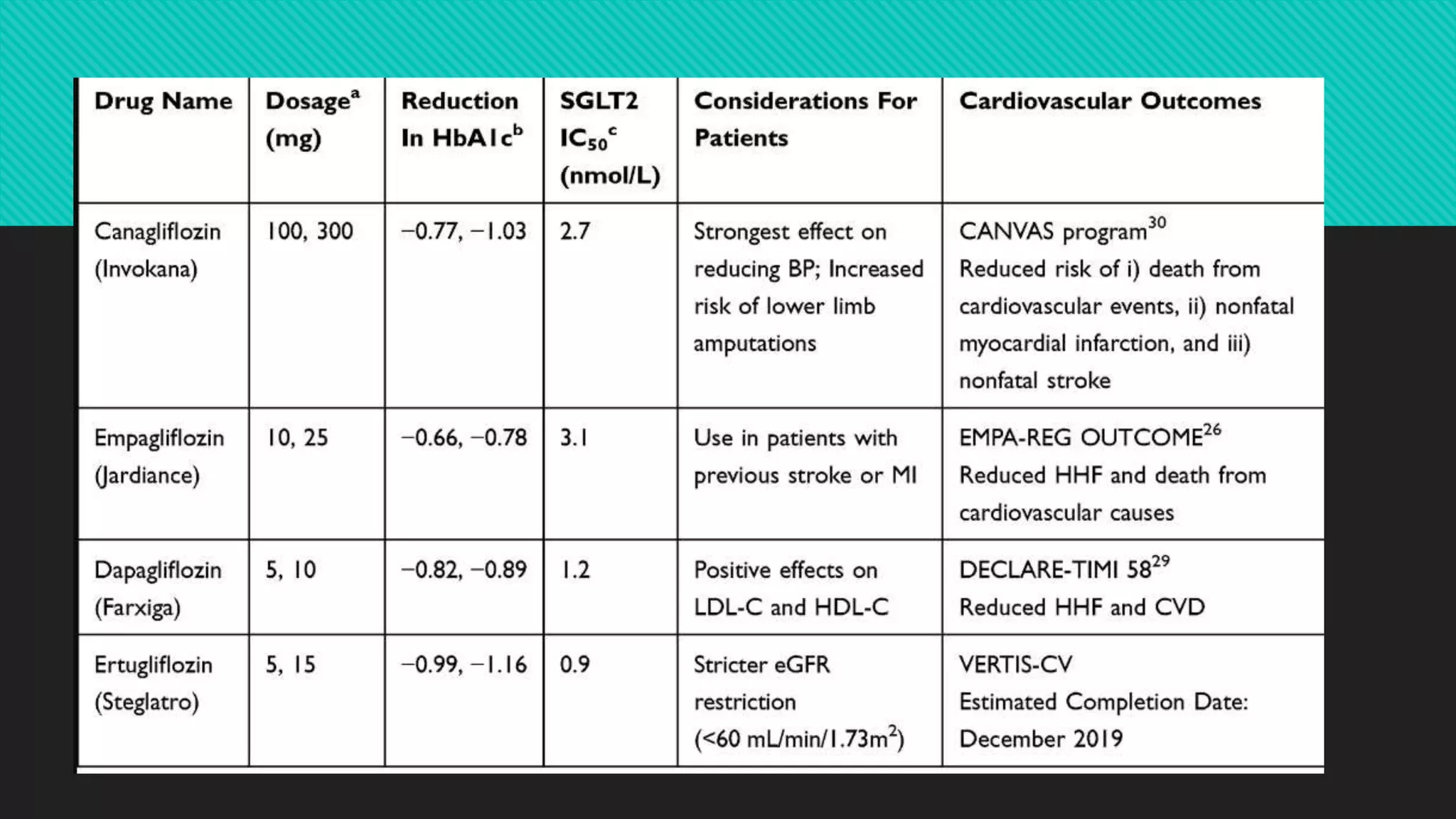
The document summarizes clinical trials evaluating SGLT2 inhibitors:
1) The EMPA-REG trial found that empagliflozin reduced the risk of cardiovascular death, hospitalization for heart failure, and all-cause mortality compared to placebo in patients with type 2 diabetes at high cardiovascular risk.
2) The CANVAS trial found that canagliflozin reduced the risk of major adverse cardiovascular events and hospitalization for heart failure compared to placebo in patients with type 2 diabetes at high cardiovascular risk.
3) The DECLARE-TIMI 58 trial found that dapagliflozin did not increase the risk of major adverse cardiovascular events compared to placebo in patients with type 2 diabetes


![ROLE OF SGLT2 AND SGLT1 IN RENAL
AND GI GLUCOSE TRANSPORT AND THE
EFFECTS OF DIABETES
In the kidneys, glucose is freely filtered by the glomeruli
Glucose reasorption (99% of filtered) occurs in PCT by active transport (SGLT1/2)
Approximately, 90% by the high capacity, low affinity SGLT2 with the low capacity, high affinity SGLT1
transporter, in the distal segment, responsible for the remaining 10%.
These transporters bind with both sodium ions (Na+) and glucose in the tubular filtrate, then these are
translocated across the cell membrane.
This process is driven by the electrochemical Na+ gradient between the tubular filtrate and the
intracellular space and is called secondary active-transport
Glucose in the tubular epithelial cell is then transported down a concentration gradient across the
basolateral membrane to the systemic circulation by GLUT2.
When the blood glucose concentration rises above about 10 mmol/L, filtered glucose load exceeds the
tubular maximum reabsorptive capacity (TmG, approximately 375 mg/min [425 g/d] in healthy individuals)
excess glucose is excreted in the urine.](https://image.slidesharecdn.com/sglt2-201015151152/75/Sglt2-inhibitors-past-present-and-future-3-2048.jpg)