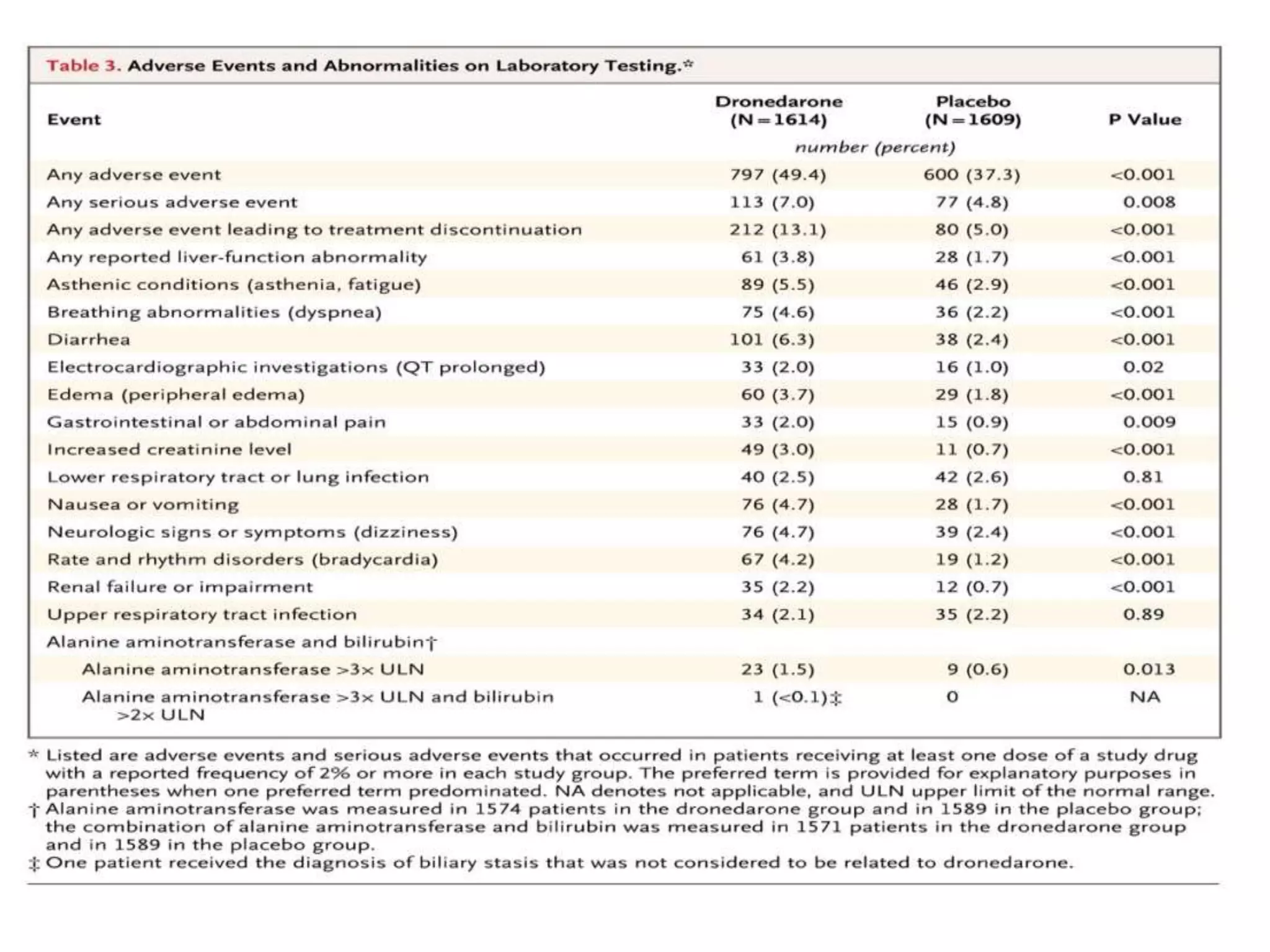
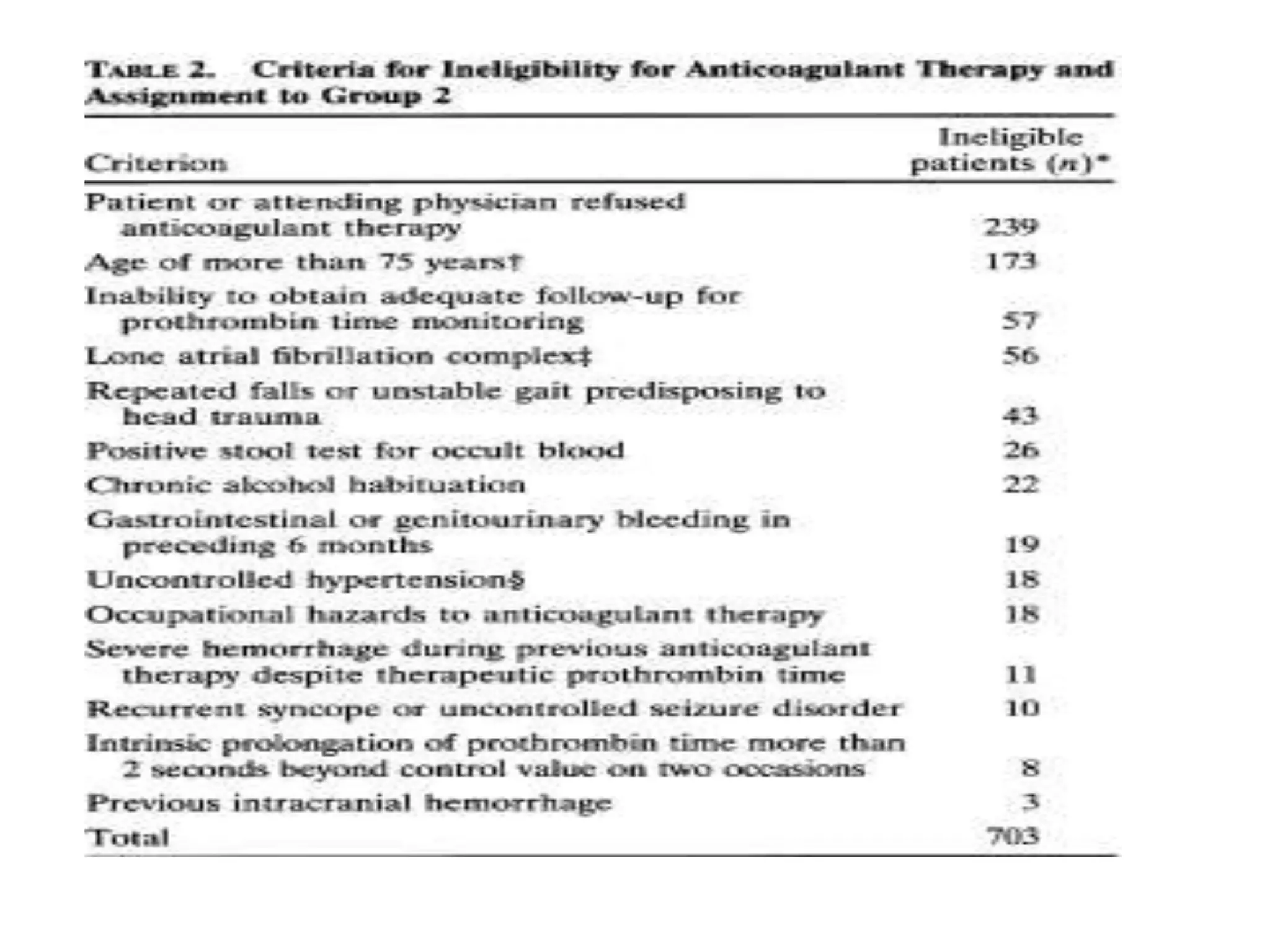
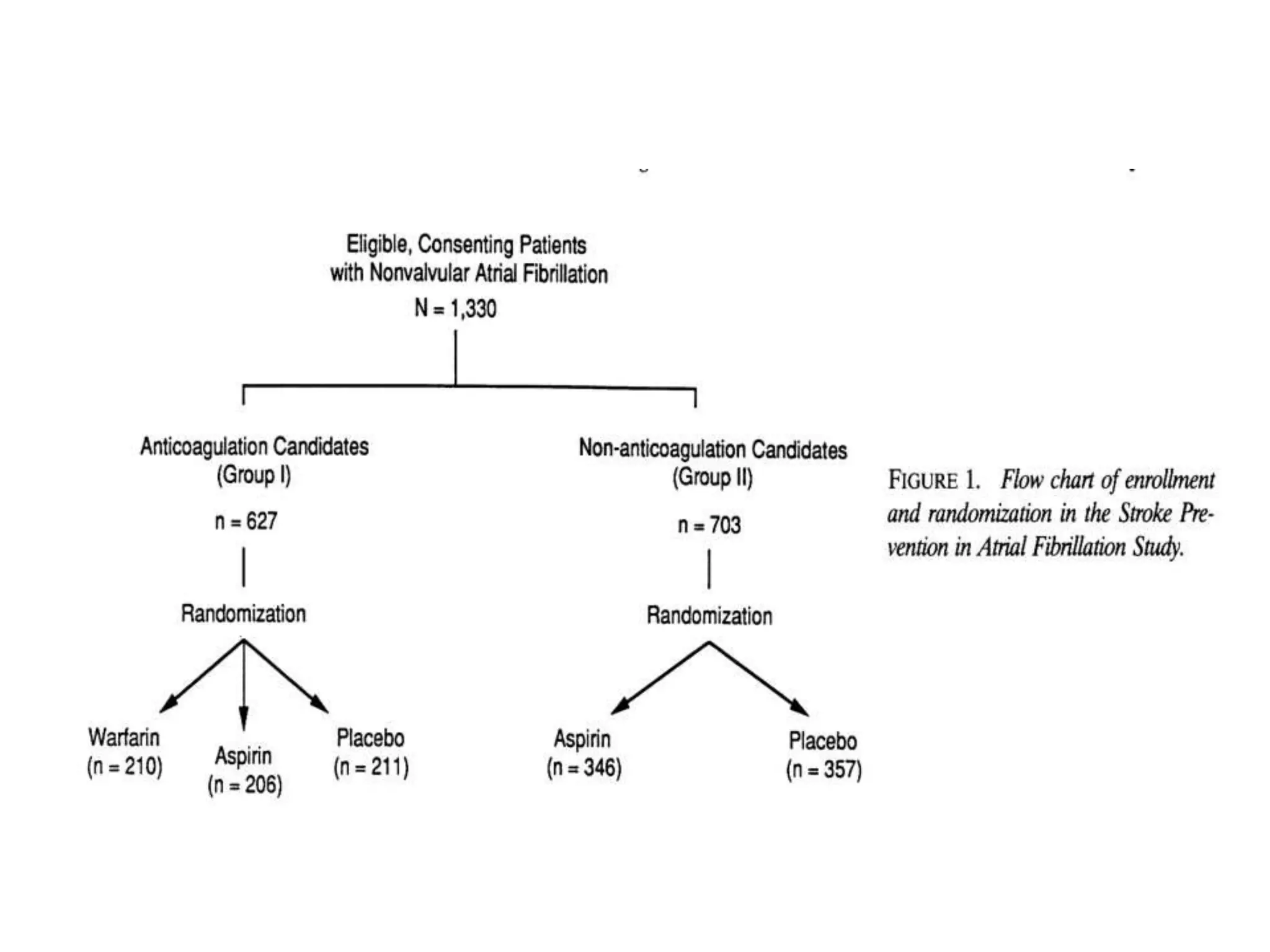
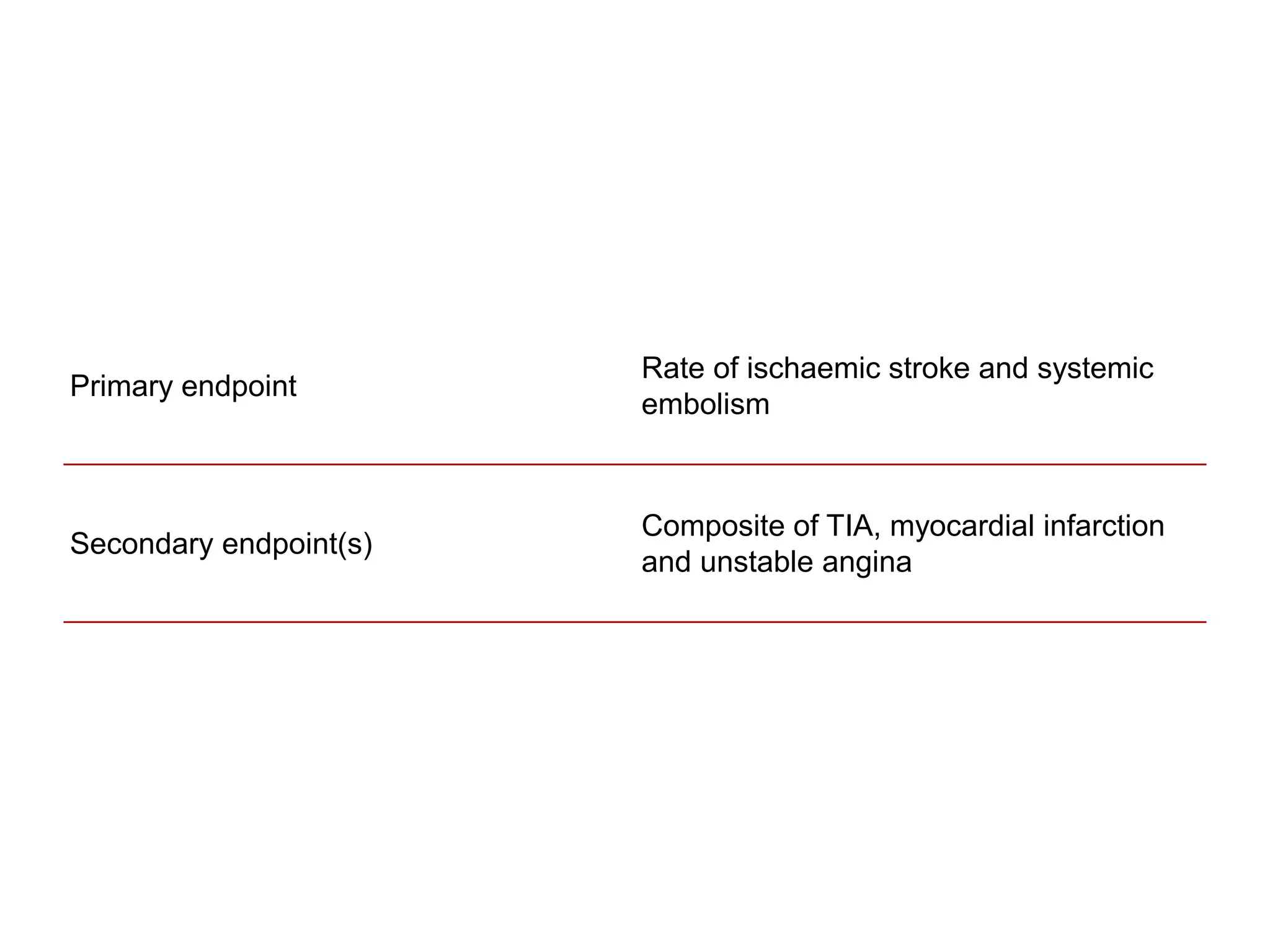
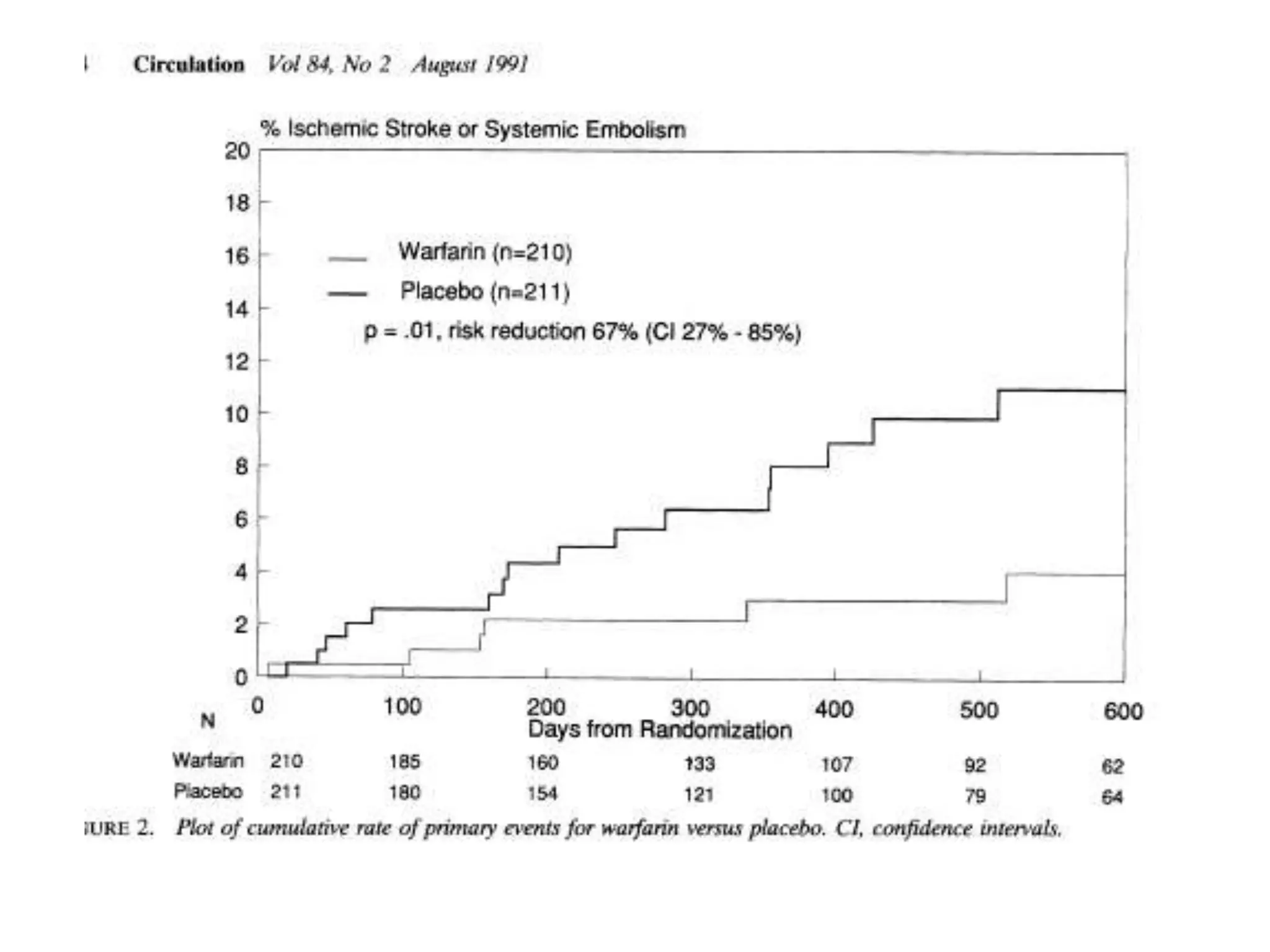
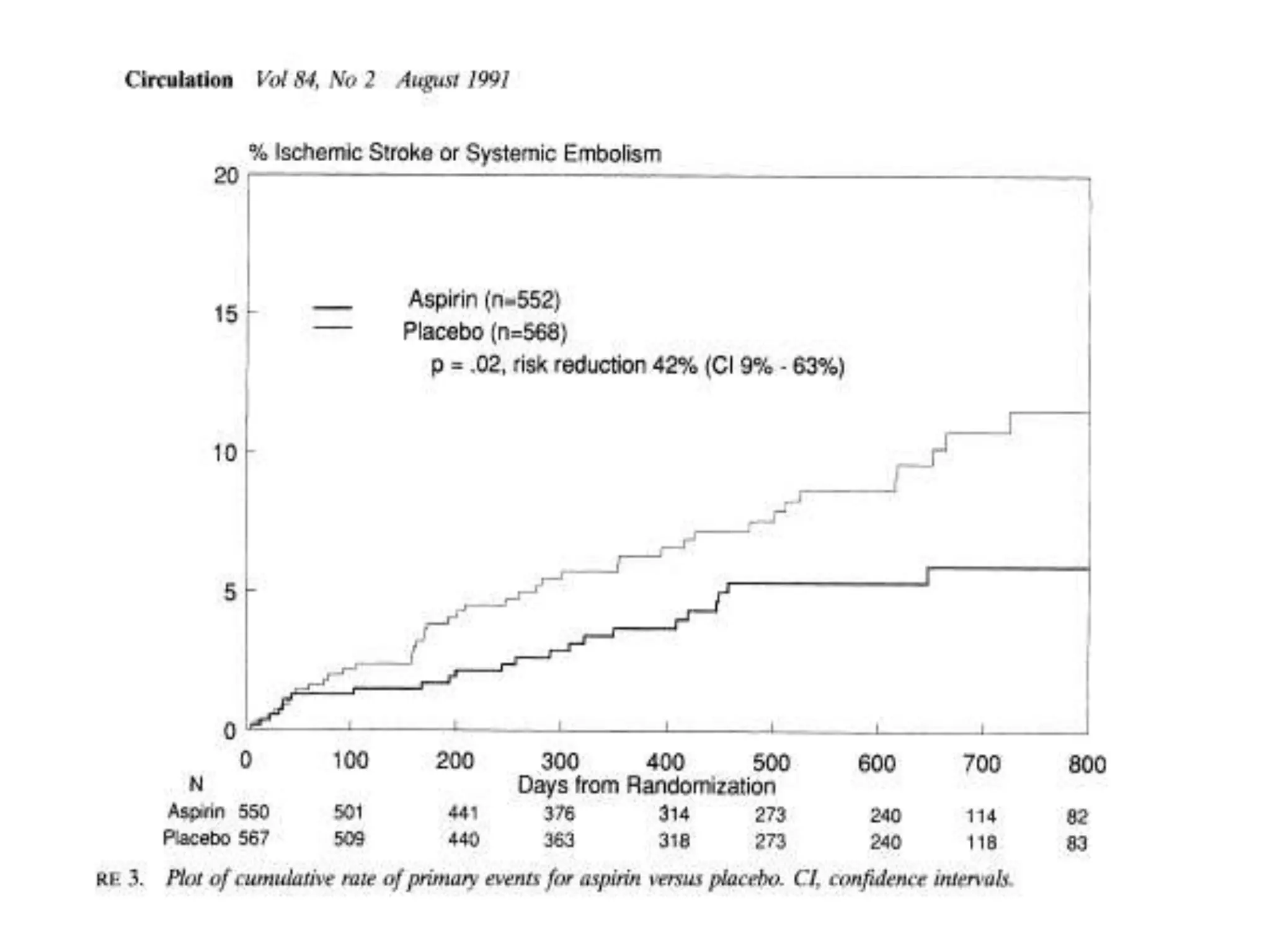
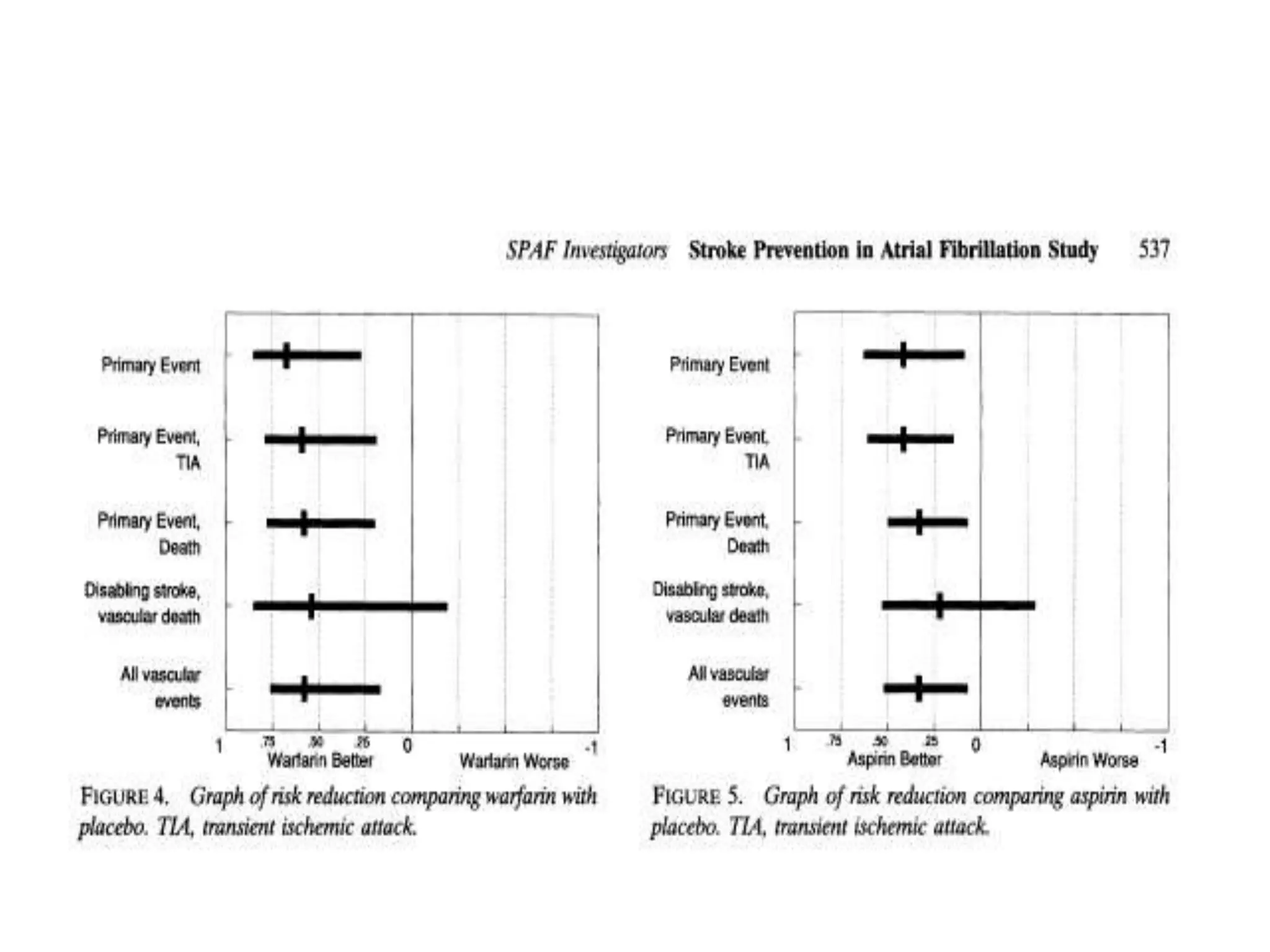
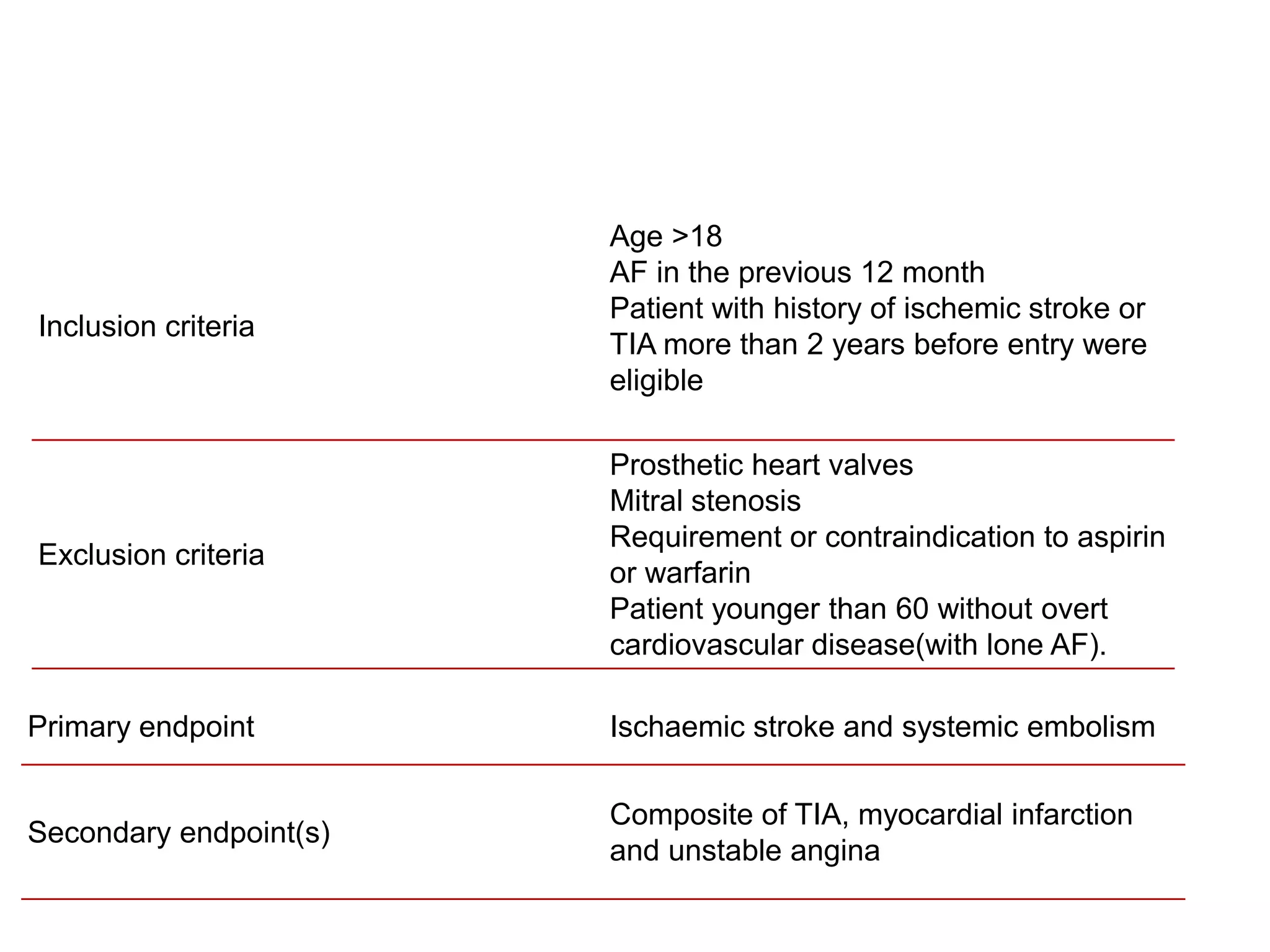
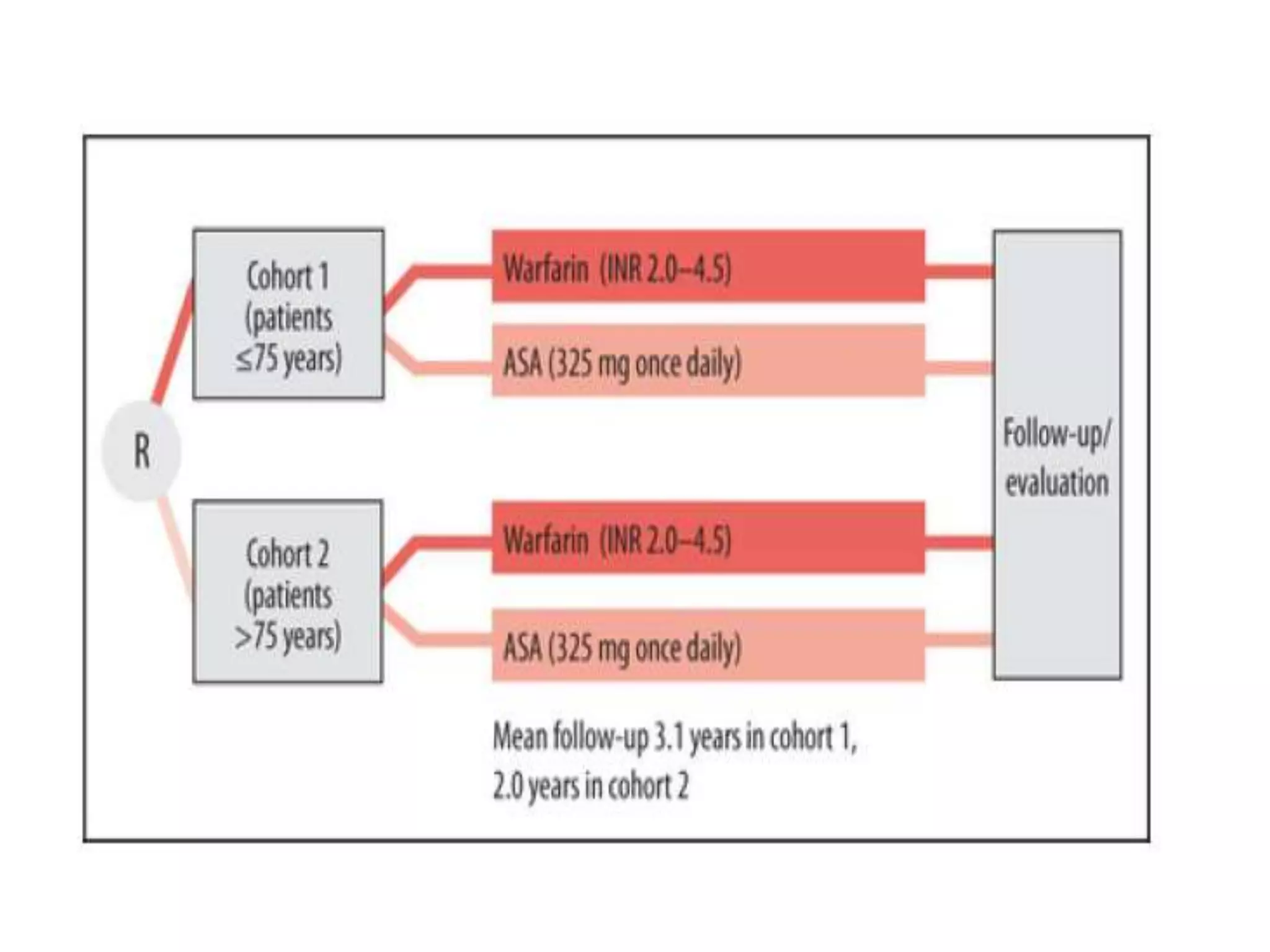
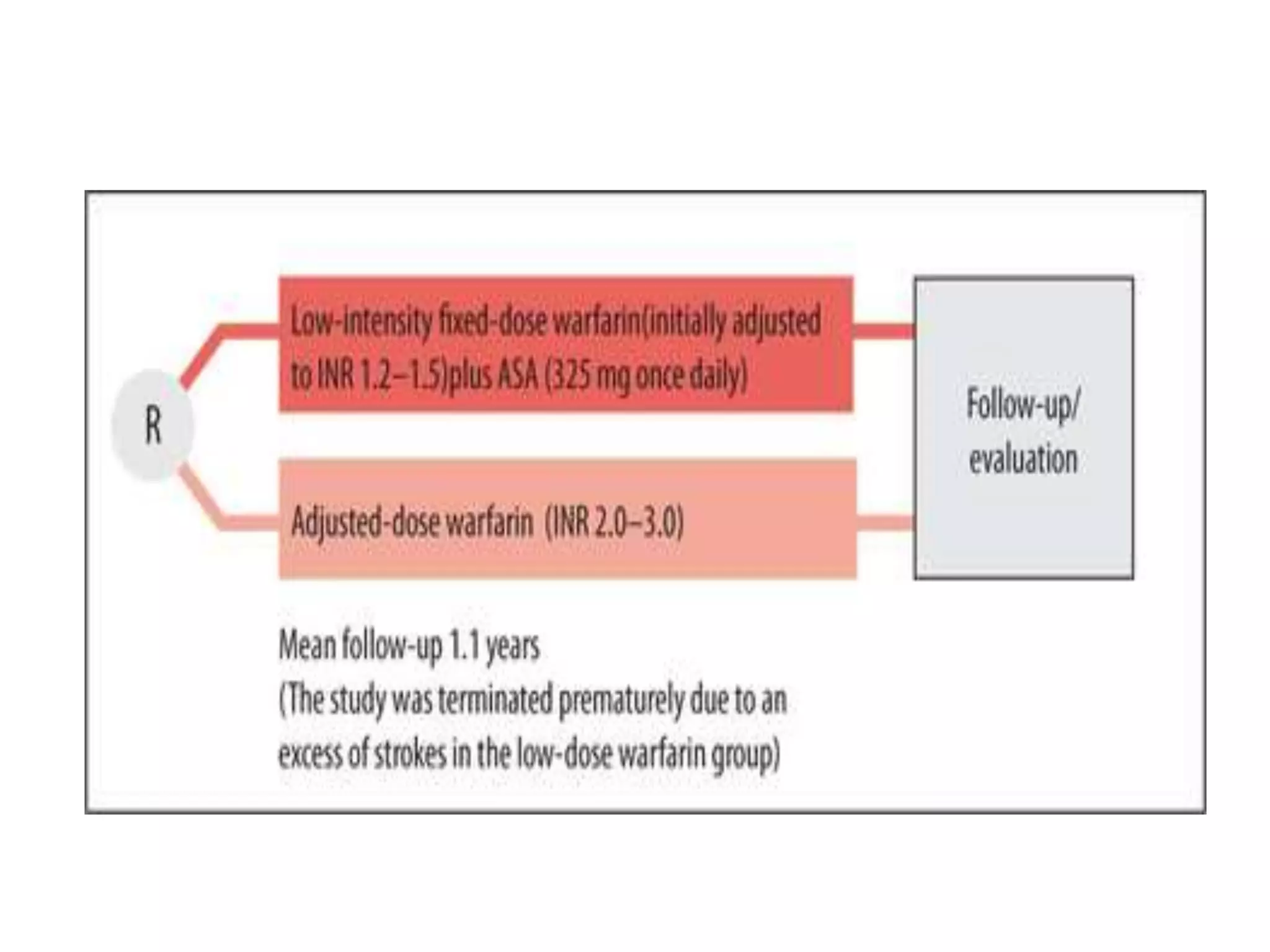
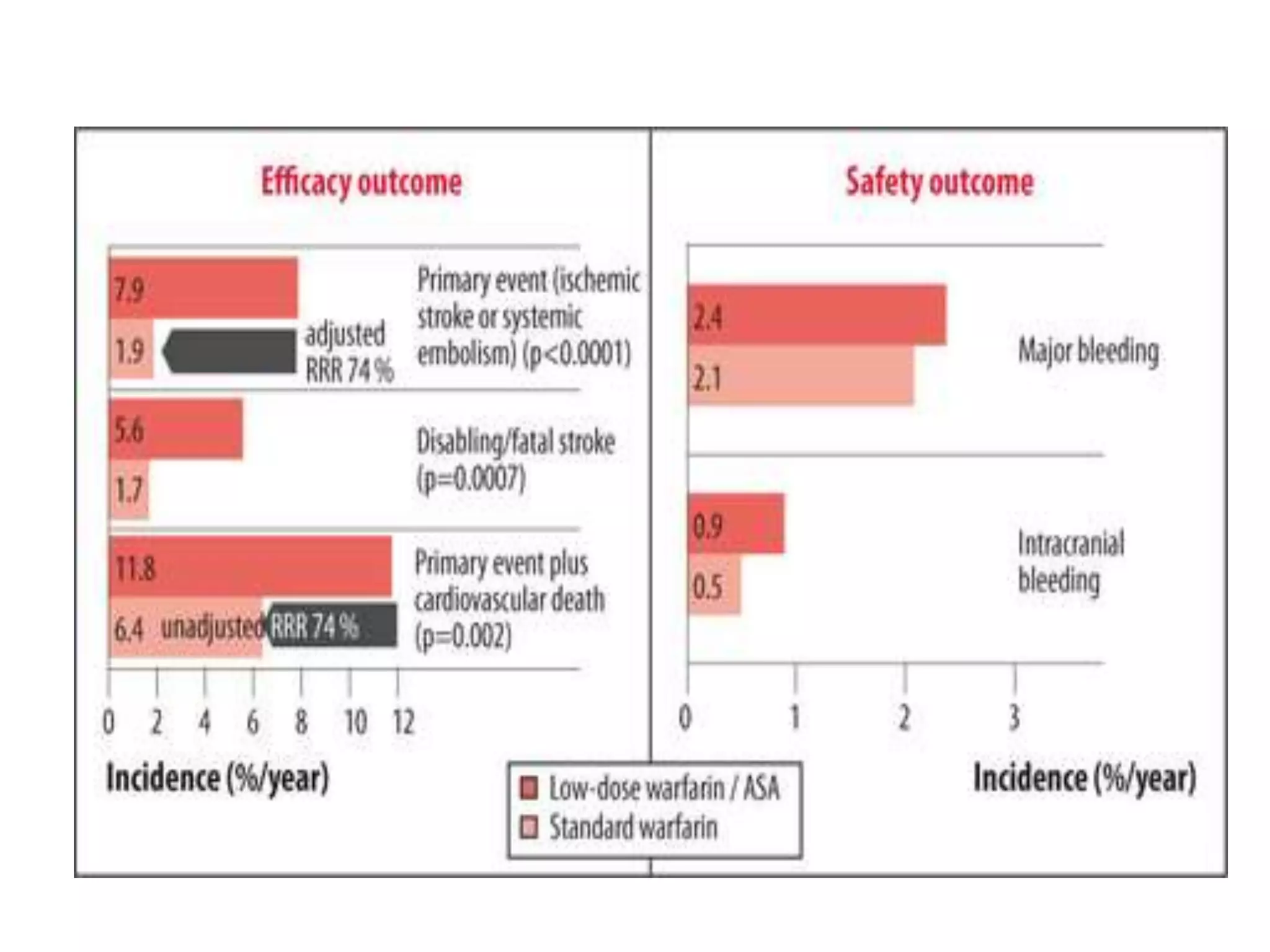
The document describes several clinical trials related to atrial fibrillation (AF) management:
1. AFFIRM trial compared rate control vs rhythm control strategies and found no difference in mortality but rhythm control had better symptom control.
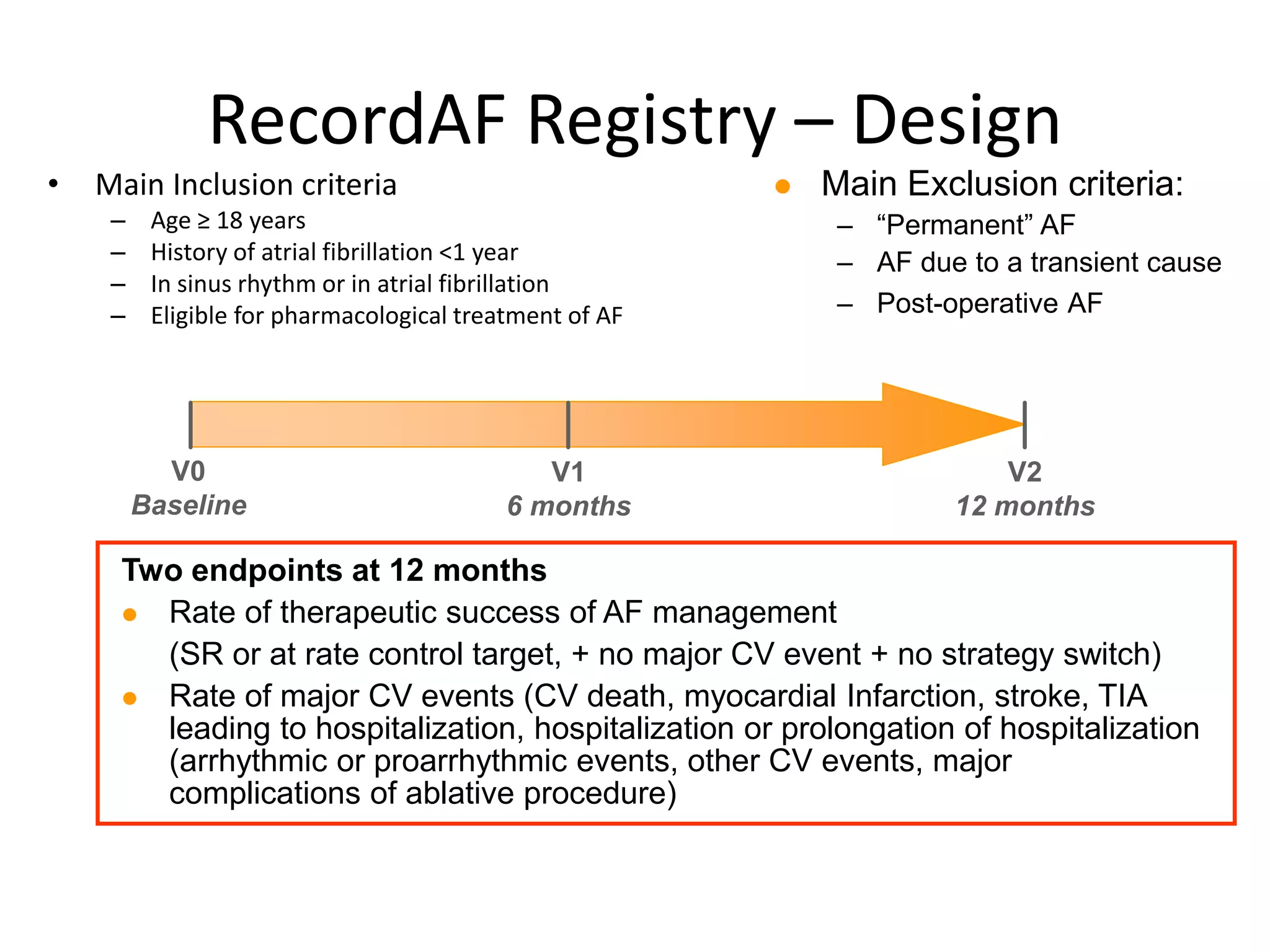
2. RecordAF registry found in real-world practice, rhythm control was preferred and had better therapeutic success rates than rate control.
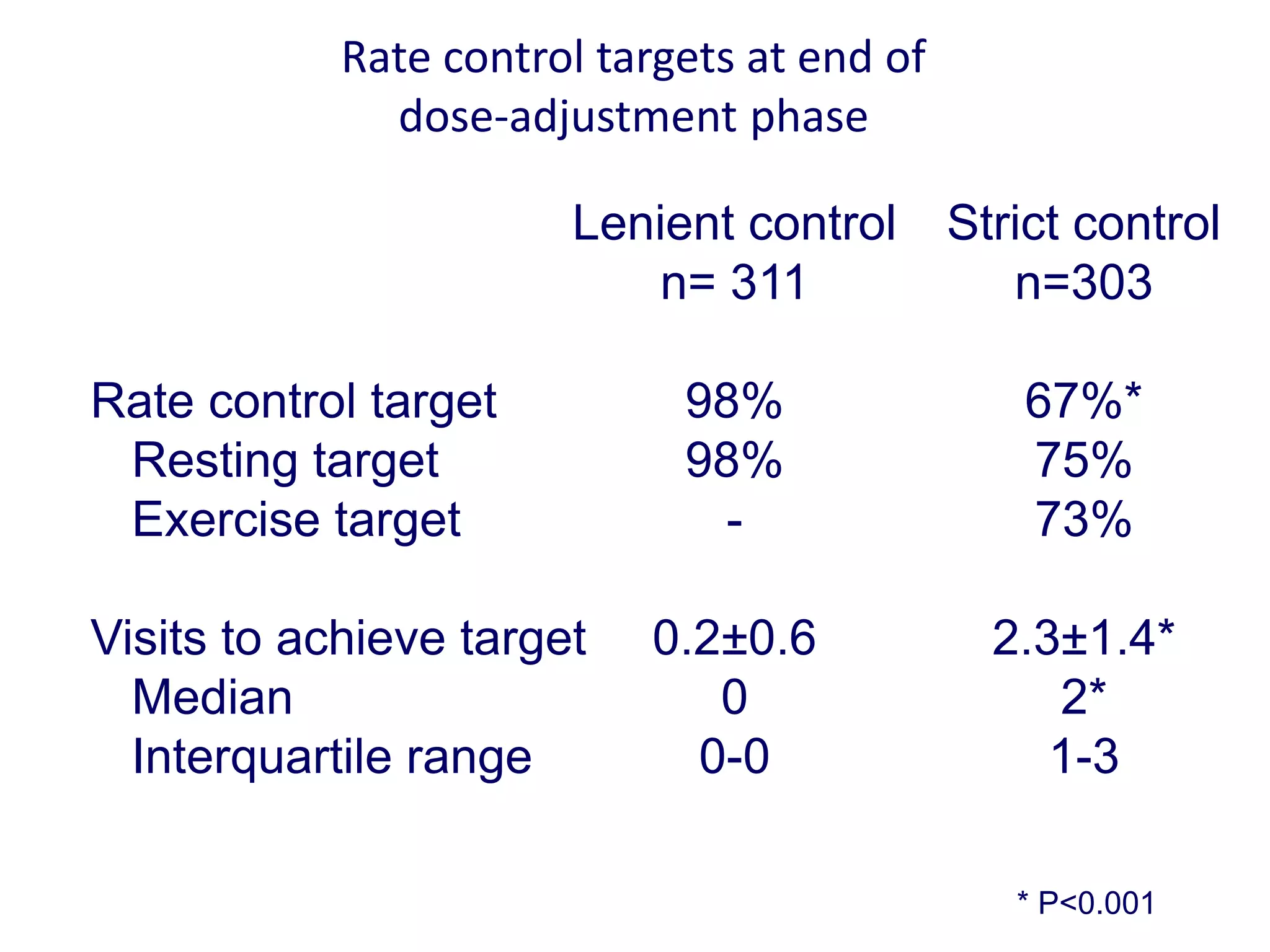
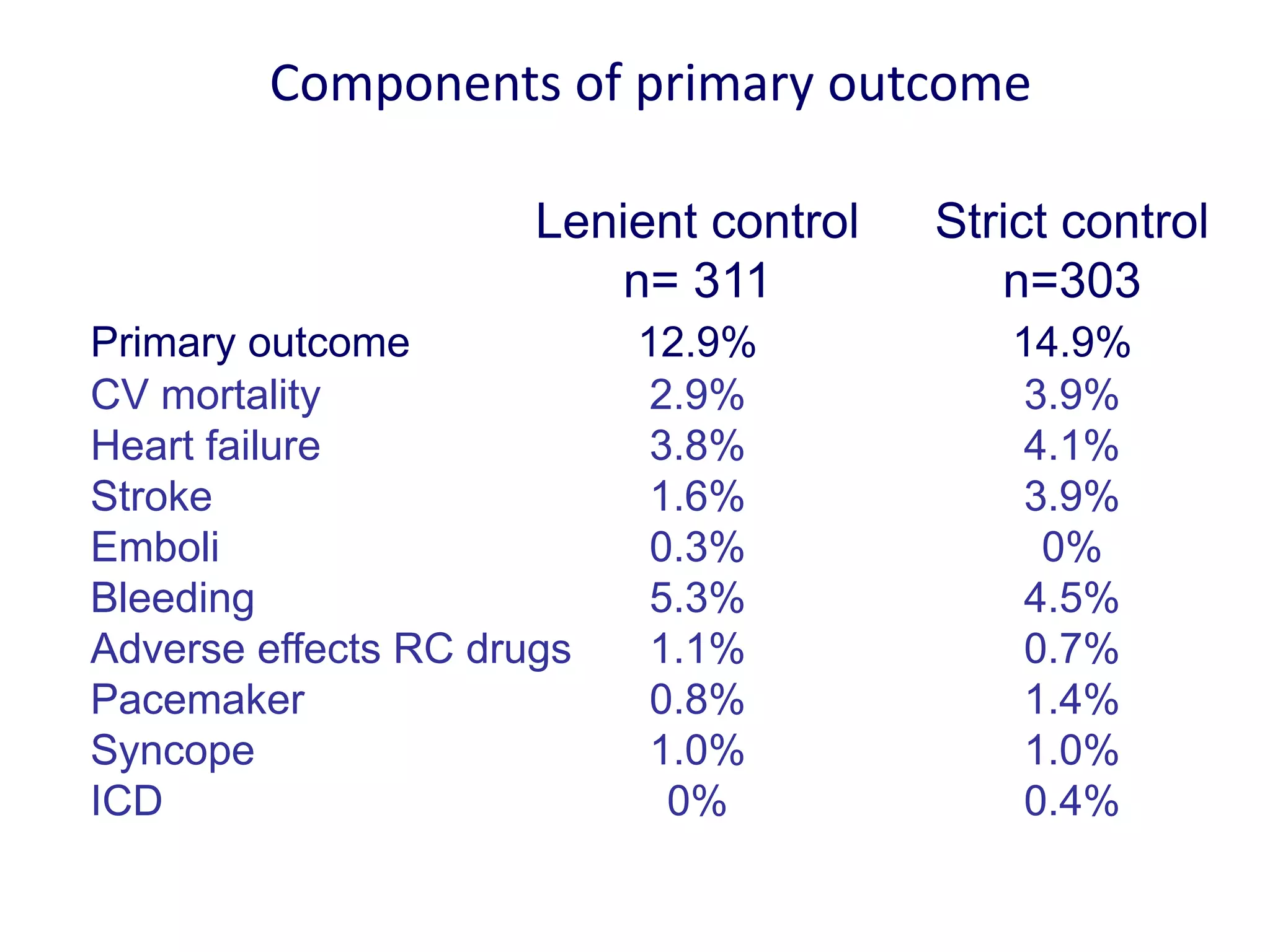
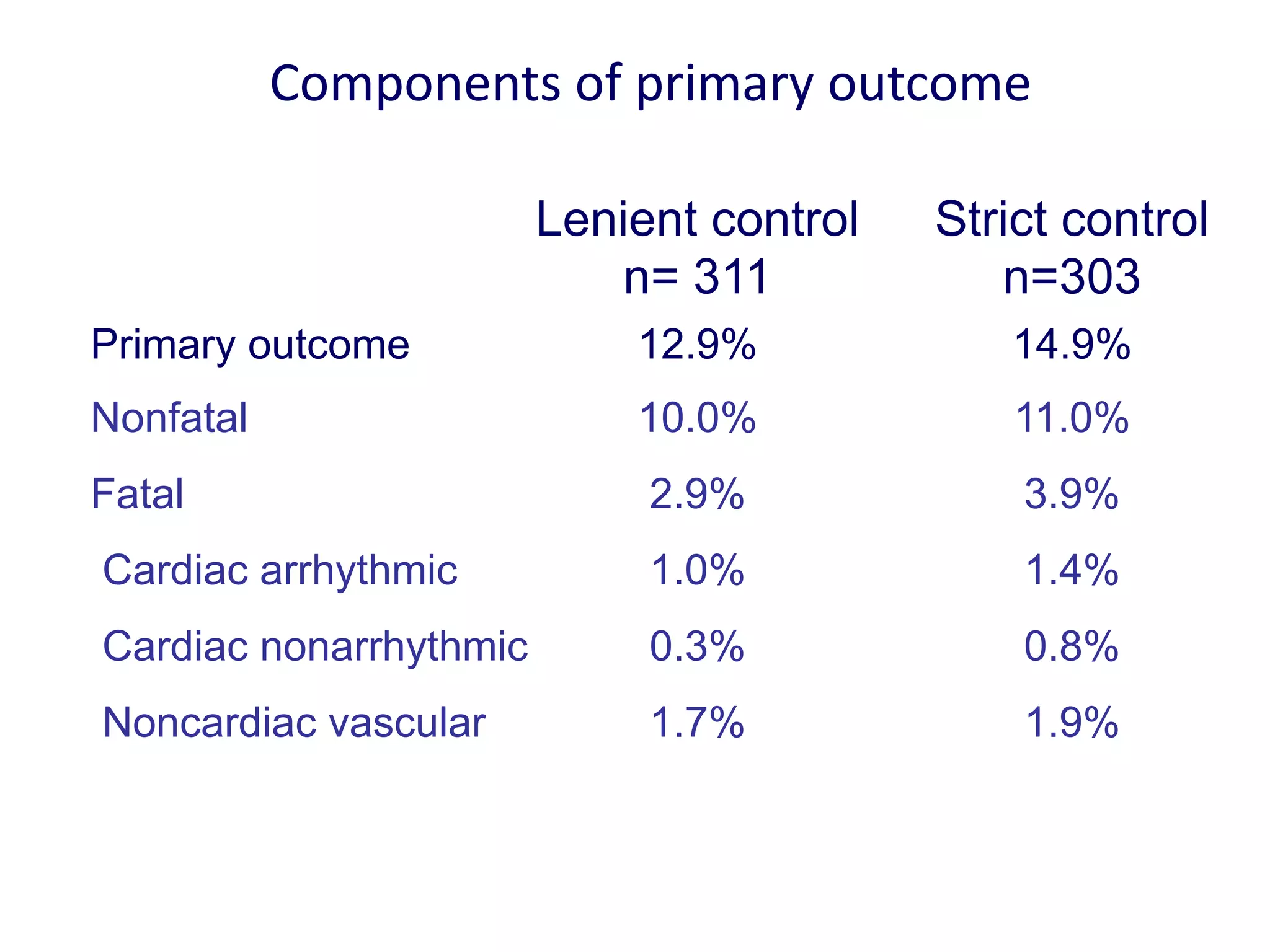
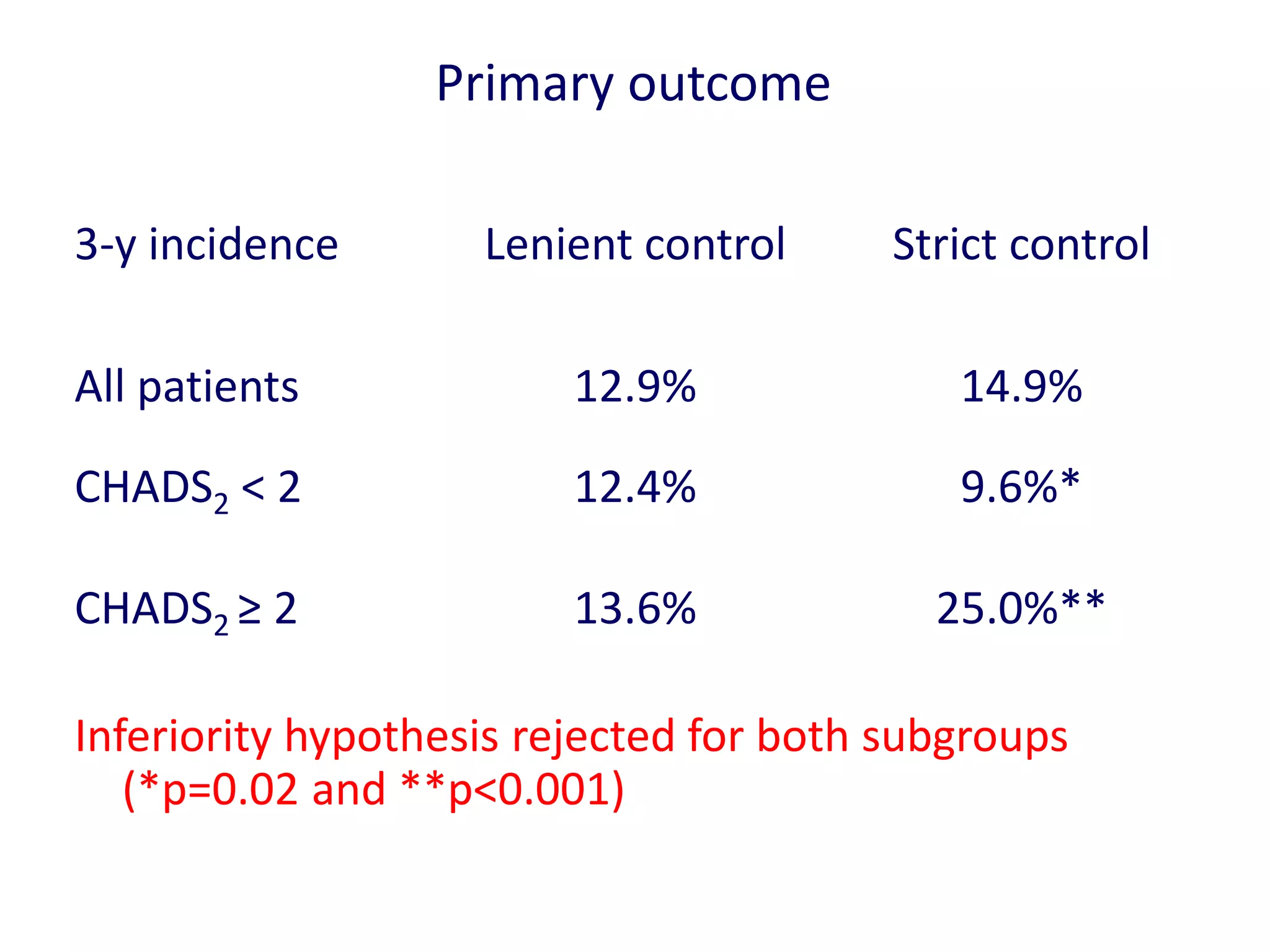
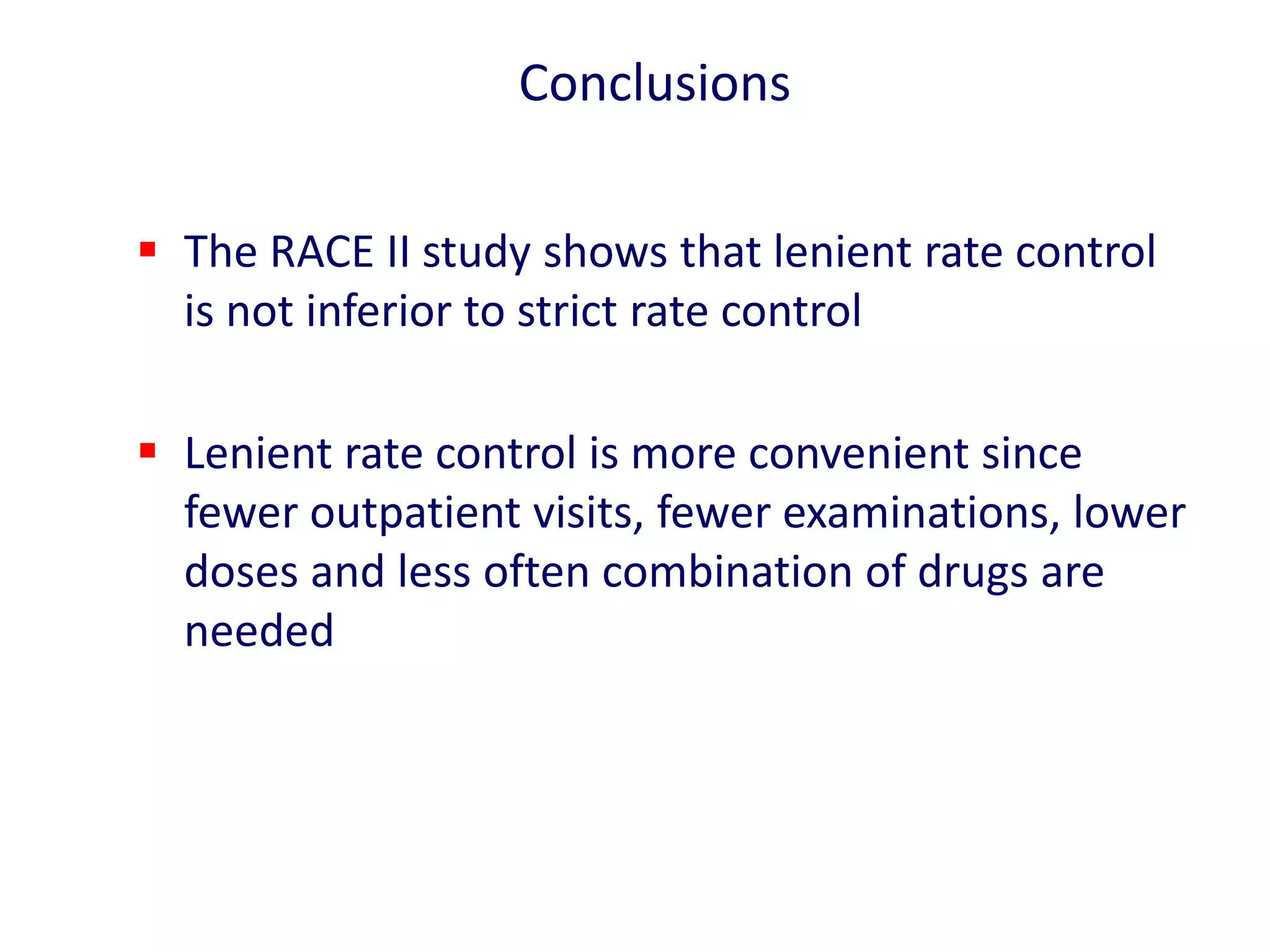
3. RACE II trial showed lenient rate control (HR<110bpm) was not inferior to strict control (HR<80bpm) for cardiovascular outcomes in permanent AF.
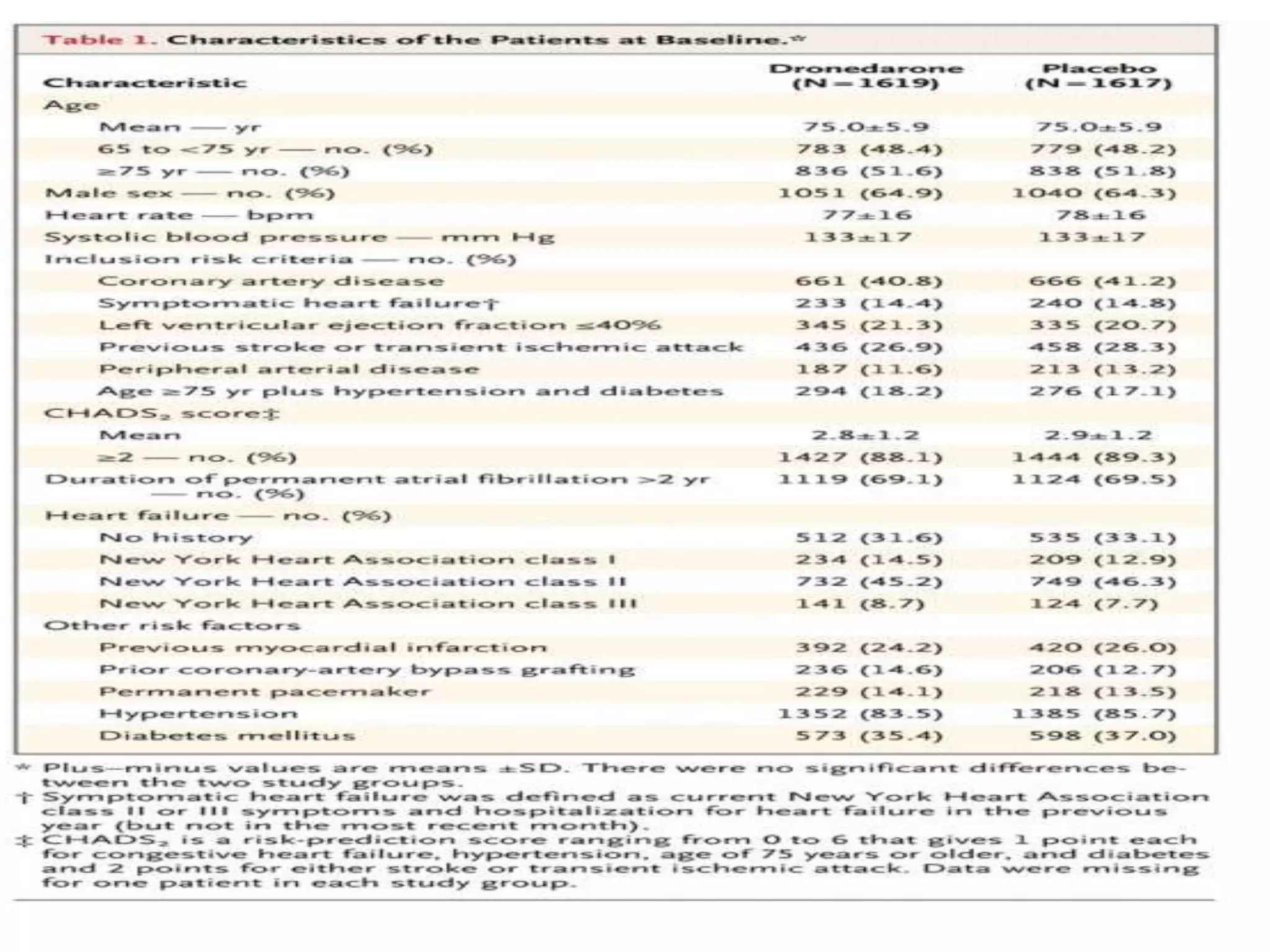
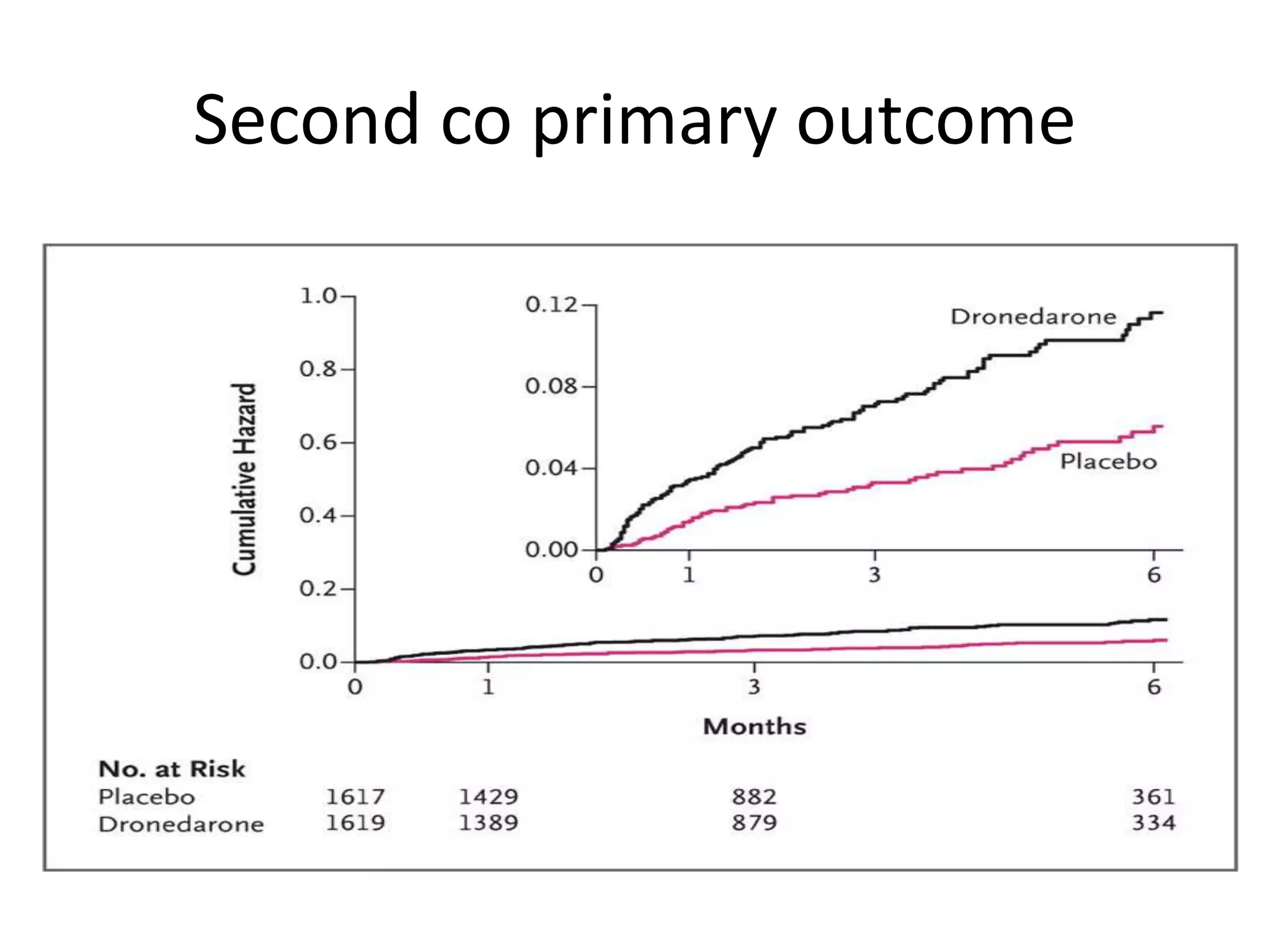
4. ATHENA trial found dronedarone reduced cardiovascular hospitalizations in AF patients at high risk of events.




































































![• Rivoraxaban 20 mg daily
• Warfarin with dose adjusted with INR [2-3]](https://image.slidesharecdn.com/aftrials-170511175143/75/Af-trials-94-2048.jpg)







